March 7, 2012 — The U.S. Food and Drug Administration (FDA) cleared the Abbott Absolute Pro vascular self-expanding stent system for use in iliac artery peripheral artery disease (PAD) lesions.
This device is indicated for improving luminal diameter in patients with de novo or restenotic atherosclerotic lesions in the native common iliac artery and native external iliac artery with reference vessel diameters between 4.3 and 9.1 mm and lesion lengths up to 90 mm.
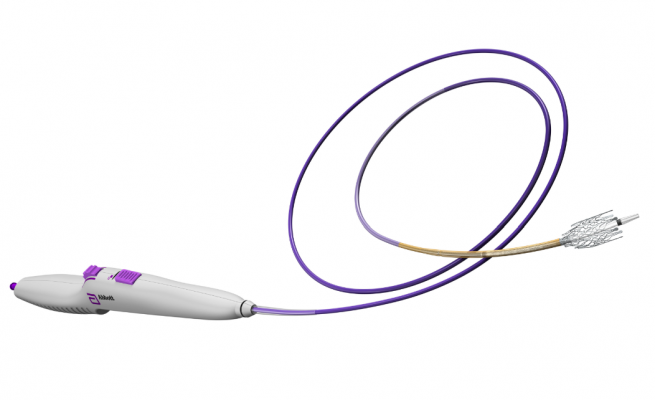
The self-expanding nickel titanium stent is pre-mounted on an over-the-wire (OTW) delivery system. The stent delivery system is compatible with a 0.035" (0.89 mm) guidewire and comes in lengths of 80 and 135 cm. The Absolute Pro stent system is available in diameters of 6-10 mm in 1 mm increments. The stent comes in lengths of 20, 30, 40, 60, 80, and 100 mm.
A total of 12 markers made of a radiopaque nickel-titanium alloy are located at the ends of the stent.
The delivery catheter is comprised of a retractable sheath that covers the stent during delivery and a radiopaque tip. Rolling back the thumbwheel on the delivery system handle deploys the stent. The locking mechanism is located on top of the handle.
The Absolute Pro has been commercially available in the United States as a biliary stent since September 2008. The same device labeled as the Absolute Pro Peripheral Stent System is commercially available in more than 80 countries outside the United States.
For more information: www.abbottvascular.com

