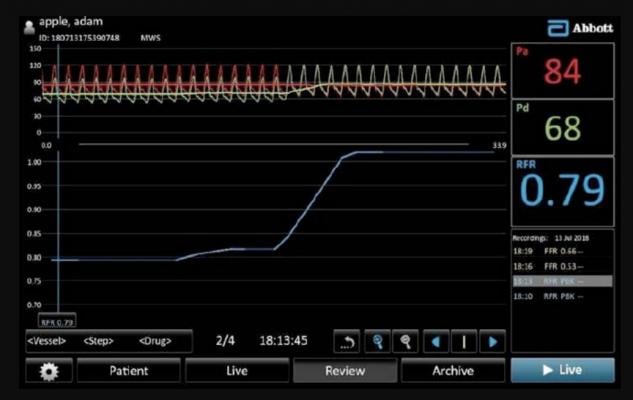
Abbott's Resting Full-cycle Ratio (RFR) intravascular diagnostic test is a newer type of fractional flow reserve (FFR) physiologic assessment. RFR is used to help identify significant narrowings of coronary arteries to determine the need for a stent or if a patient can be treated medically.
March 5, 2019 – The U.S. Food and Drug Administration (FDA) cleared Abbott's Resting Full-cycle Ratio (RFR) intravascular diagnostic test to identify significant narrowing of coronary arteries to determine the need for a stent or if a patient can be treated medically.
The standard technology to assess severity of coronary lesions is fractional flow reserve (FFR), which requires the injection of vasodilator drugs to induce pharmacologic stress. Physicians can use the RFR diagnostic test to detect the severity of coronary artery stenosis at rest, without administering potentially costly stressor agents like adenosine, which causes patient discomfort and to lengthens procedure time.
“Accuracy in identifying the severity of arterial narrowing or blockages is critical in guiding if and when a stent should be implanted, avoiding unnecessary procedures,” said Ziad A. Ali, M.D., director of intravascular imaging and physiology at Columbia University Medical Center's Center for Interventional Vascular Therapy. “Abbott’s RFR provides interventionalists with a new, effective method of analysis that identifies important stenoses with the least discomfort, offering a better experience for patients.”
Late-breaking trial results from the RE-VALIDATE study, an all-comers prospective analysis of retrospective real-world hospital data, were presented yesterday at the Cardiovascular Research Technologies (CRT) 2019 conference in Washington, D.C. During the study, RFR demonstrated equivalence against instantaneous wave-free ratio (iFR), an older version of resting coronary artery stenosis severity assessment, with overall diagnostic accuracy of 97.8 percent.[1]
The RFR technology also offers capabilities to determine the most significant blockage in arteries with multiple lesions and offers improved security with full encryption of patient data. Additionally, it will be used with the new wireless PressureWire X, which is uniquely designed for confident and accurate physiology measurements.
Find more information, including U.S. safety information.
For more information: www.abbott.com
References:
1. Kumar, G, et al. Real world validation of the Resting Full-cycle Ratio (RFR): A novel non-hyperemic index of coronary artery stenosis severity. Presented at CRT 2019, Washington D.C.


 November 14, 2025
November 14, 2025 









