May 17, 2012 - Philips Healthcare announced the availability of CardioCare Wireless Arrhythmia Services, the latest addition to the company’s remote diagnostic arrhythmia and remote patient monitoring portfolio. This new service, available only in the United States, is designed to streamline the complex process of remotely monitoring cardiac patients and capture critical information sooner.
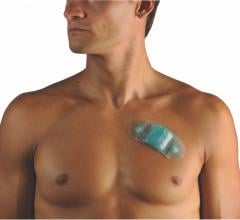
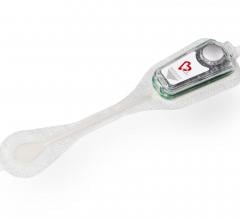
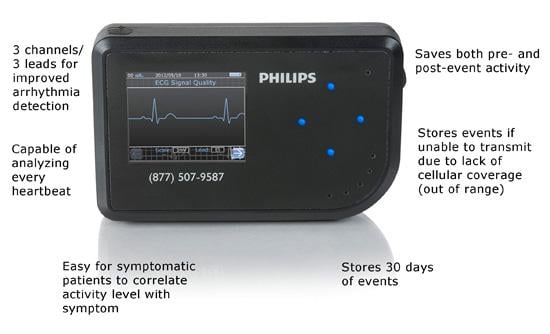
With Philips CardioCare, patients wear a one-piece monitor with an intuitive interface designed to help improve compliance. Using this device, patients’ heartbeats are transmitted wirelessly to highly qualified cardiac technicians at the Philips Clinical Evaluation Center, who can analyze them and alert cardiologists to critical patient data. This capability is an evolution beyond previous monitors, which transmitted patient data over telephone lines. As part of its portfolio of services, Philips distributes the wireless monitor made by TZ Medical. The monitor received U.S. Food and Drug Administration (FDA) marketing clearance last year.
“The CardioCare monitor uses advanced device technologies from TZ Medical Inc. including Monebo’s robust arrhythmia detection algorithms,” said John Lubisich, president, TZ Medical Inc. “Pairing this latest technology with Philips’ experience in the cardiac monitoring space helps simplify the process of remote monitoring for physicians.”
Philips’ helps cardiologists remotely monitor patients for a range of arrhythmias and gives them the ability to select the appropriate type of monitoring for each patient. For symptomatic patients, CardioCare is designed to help them link their symptoms to their activity level. For patients who do not experience symptoms, CardioCare can automatically recognize and record cardiac events. In either mode, the monitor will transmit recorded events wirelessly back to the Philips call center without patient interaction.
“CardioComm Solutions provides a customized software solution that seamlessly integrates CardioCare into the Philips Evaluation Center,” said Etienne Grima, chief executive officer of CardioComm Solutions Inc. “This provides Philips’ technicians with complete cardiac data they can then translate into clear, actionable insights for doctors.”
“As the global leader in hospital cardiac monitoring, Philips strives to improve the lives of patients by simplifying the patient experience and enabling timely diagnoses of cardiac arrhythmias,” said Mark Szewczyk, general manager, Philips Remote Patient Monitoring. “CardioCare is a great addition to our portfolio — it allows us to combine smart innovations with our experience in cardiology to meet clinician and patient needs.”
For more information: www.philips.com

 July 24, 2024
July 24, 2024