Medtronic Inc.
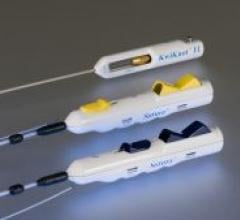
Medtronic Inc. will showcase its Export Aspiration Catheter at TCT 2008. Recent results from an…
Medtronic Inc. recently announced the initiation of the first investigational sites in the U.S…
The Mynx VCS vascular closure system (VCD) by AccessClosure designed to seal a puncture site in…
Accumetrics develops and manufactures the VerifyNow System, a comprehensive system for the…
Neovasc (formerly Angiometrx) is the developer of the Metricath System, an arterial and in-stent…
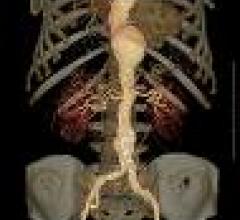
Bracco's Isovue (iopamidol injection) X-ray/CT contrast is reportedly a safe and effective…
St. Jude Medical Inc. received European CE Mark approval and FDA clearance for the Epicor LP…
FiatLux Imaging Inc.
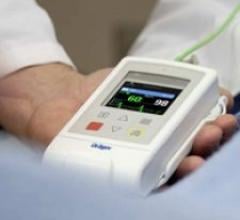
Draeger Medical Inc.
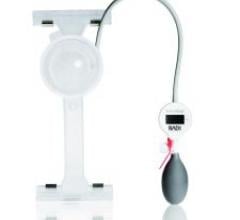
RADI Medical Systems Inc. offers its line of FemoStop Femoral Compression Systems that are now…
The Sorin Group offers the REPLY family of dual and single chamber pacemakers, with the world’s…
Siemens Medical Solutions will showcase its recently FDA-cleared SOMATOM Definition AS, said to…
OrbusNeich will showcase its Genous Stent at TCT 2008, a stent that was CE Marked in 2005.
XTENT Inc.'s Custom NX stent system reportedly allows physicians to customize the length and…
Toshiba’s Infinix VF-i SP with Type S Processor includes a digital processor that will expand on…
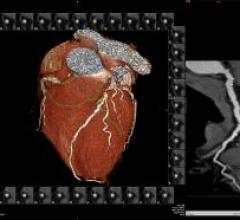
Vital Images Inc. offers the ViTAL Enterprise Solution, which is designed to provide customers…
The Radpad Femoral-Entry Angiography Shield for coronary, Radpad Peripheral Drape for peripheral…
GE Healthcare will highlight new capabilities for its Innova series of interventional X-ray…

 August 25, 2008
August 25, 2008