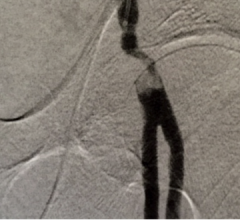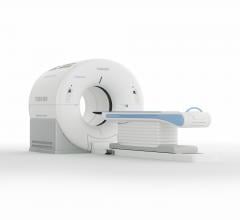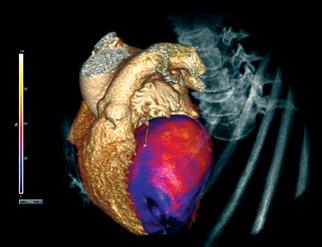
Cardiac computed tomography angiography (CCTA) is known to have excellent anatomical imaging, but has lacked the ability for functional assessments, requiring chest pain patients with intermediate stenosis lesions to be sent to the cath lab or nuclear myocardial perfusion imaging. However, recent advances in CCTA image analysis software and the accumulation of supporting clinical data may soon enable CT perfusion imaging and virtual fractional flow reserve-CT (FFR-CT) to become mainstream in the coming years.
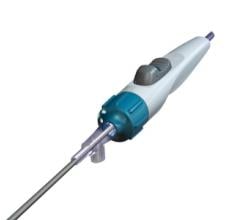
C. Dorn Smith, M.D., vascular surgeon in Kingstree, South Carolina, was successful in using a new device to remove blood clots from a patient with a cold leg using the Aspire mechanical thrombectomy device.
Biotronik announced the publication of promising clinical results regarding its Pulsar-18 stent platform. Adding to the similarly encouraging data collected in the earlier 4EVER and PEACE trials, the new results — published in Clinical Medical Insights: Cardiology — further confirm the safety and efficacy of the Pulsar-18 self-expanding nitinol stent, with positive patency results for very challenging cases of femoropopliteal disease.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Cordis Corp. announced the launch of the new Elitecross support catheter in the United States and Outback Elite re-entry catheter in the United States, Europe and Japan. This expands the Cordis Crossing Portfolio for treatment of chronic total occlusions (CTO), a suite of specialty and workhorse solutions designed to support physicians in crossing the most complex lesions.
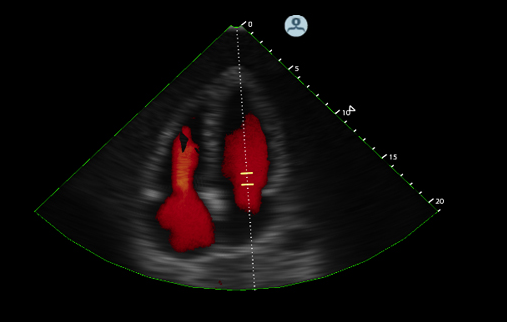
More than 50 companies and organizations will display their latest products and services at the American Society of Echocardiography's (ASE) 26th Annual Scientific Sessions, June 12-16 at in Boston, Mass. ASE 2015 is the world's premier meeting for cardiovascular ultrasound practitioners, and promises a wealth of cutting-edge education, research, and the latest vendor technology.
The nation's top consumer, patient and labor advocates recently praised the proposed Stage 3 Meaningful Use rules, describing how they would help ensure health information technology (IT) meets the needs of patients and families.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
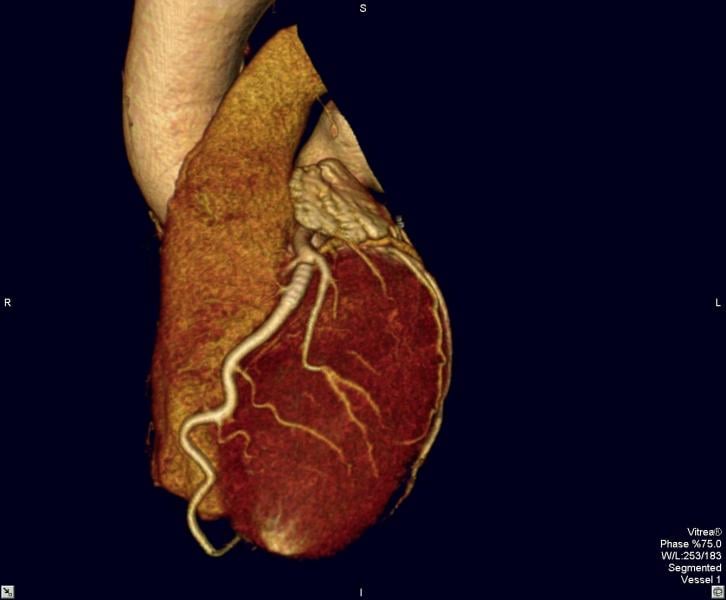
AstraZeneca announced full results from the PEGASUS-TIMI 54 study, a large-scale outcomes trial investigating Brilinta (ticagrelor) tablets plus low dose aspirin, at the American College of Cardiology (ACC) 64 th annual scientific session and expo in March. The study compared Brilinta to placebo plus low dose aspirin, for the chronic secondary prevention of atherothrombotic events in patients who had experienced a heart attack one to three years prior to study enrolment. Study results were simultaneously published in the New England Journal of Medicine online.
University Hospitals Case Medical Center physicians in the Harrington Heart & Vascular Institute were the first in Ohio to implant a new device to treat right ventricular heart disease.
Professional physician associations consider certain routine tests before elective surgery to be of low value and high cost, and have sought to discourage their utilization. Nonetheless, a new national study by researchers at NYU Langone Medical Center finds that despite these peer-reviewed recommendations, no significant changes have occurred over a 14-year period in the rates of several kinds of these pre-operative tests.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Healthcare networks can now remotely manage computed tomography (CT) protocols and capture valuable analytics that could improve safety with Toshiba America Medical Systems Inc.'s CT Vitality software. Part of the Vitality Solutions portfolio, CT Vitality is available on Aquilion One systems and will facilitate protocol utilization and dose usage analytics so hospitals and multi-site integrated delivery networks (IDNs) can ensure consistently safe scans every time.
Start-up company GraftWorx was chosen as "Best in Show" at the Mid-Atlantic Bio Angels (MABA) 1st Pitch Life Science event, an investors' conference held in New York in June. Graftworx is a medical device company that has developed a unique peripheral vascular prosthetic bypass graft incorporating a sensor that can alert physicians when blood flow in the graft is blocked due to restenosis.
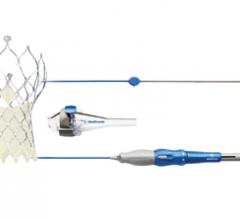
Medtronic plc announced initial clinical outcomes for its next-generation CoreValve Evolut R System at the 64th annual scientific session of the American College of Cardiology, March 14-16 in San Diego. At 30-days, the recapturable, self-expanding valve showed no incidents of all-cause mortality or stroke in a high and extreme risk patient population. The Evolut R Study enrolled 60 patients from six centers in the United Kingdom, Australia and New Zealand.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
GE Healthcare announced that it has created a solution to make positron emission tomography (PET) tracers for diagnostic imaging more readily available. At the Society for Nuclear Medicine and Molecular Imaging (SNMMI), GE launched new products for radiopharmaceutical manufacturing, including GENtrace, a small cyclotron, with a smaller footprint that is optimized for ease-of-use; and FASTlab 2, a PET tracer production module with FDG Duo cassettes, to maximize the hot cell capacity.
Kromek announced the launch of eVance, a new generation of cadmium zinc telluride (CZT)-based SPECT (single photon emission computed tomography) cameras.
Steinberg Diagnostic Medical Imaging (SDMI) of Las Vegas has bolstered its capabilities by installing the United States’ first Celesteion PET/CT system from Toshiba America Medical Systems Inc. The Celesteion allows SDMI to provide the highest quality PET/CT (positron emission tomography/computed tomography) images and exams without sacrificing patient comfort.

 June 11, 2015
June 11, 2015