Atherotech Diagnostics Lab has added three new genetic tests to its cardiometabolic test offering: warfarin sensitivity, Plavix sensitivity and the thrombophilia risk test.
The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee will review two new technologies to help treat heart failure (HF) Oct. 8-9, 2013.
Abbott promised preliminary results from a study presented at the ESC Congress 2013, suggesting that its high sensitive troponin test may help doctors improve the diagnosis and prognosis of patients presenting with symptoms of a heart attack. [1] The test could be particularly beneficial for women, who may have different presenting symptoms and are often under-diagnosed. [2] The study, which is being conducted by researchers at the University of Edinburgh, is evaluating Abbott's Architect Stat High Sensitive Troponin-I (hsTnI) test, which received CE mark in January 2013.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

The main results of the large randomized LINC study, which compared the effectiveness of the Lucas mechanical chest compression system to high quality manual chest compressions, were presented at the European Society of Cardiology Congress in Amsterdam, the Netherlands. The LINC study showed similar short-term survival rates for Lucas (23.6%) and manual (23.7%) chest compressions. At six-months, 8.5% of the patients treated with LUCAS were alive with good neurological outcomes compared to 7.6% in the manual group.
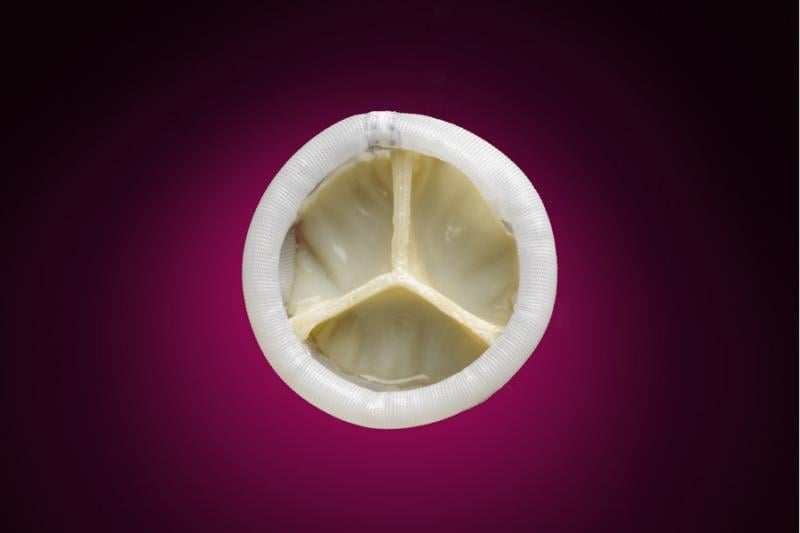
Direct Flow Medical Inc. announced the first patient enrollment in the U.S. SALUS clinical trial. The trial will study the Direct Flow Medical Transcatheter Aortic Heart Valve System, which encompasses a distinctive transcatheter aortic heart valve with a metal-free frame and flexible, low-profile delivery system designed to eliminate aortic regurgitation. The device is intended to improve the long-term survivability of aortic stenosis patients by resolving the clinical issues associated with current commercial valves.
UltraSPECT announced that MaineHealth, a family of hospitals and medical centers throughout Maine, has selected the UltraSPECT Xpress.Cardiac and Xpress3.Cardiac solutions as part of the organization’s strategy for reducing nuclear medicine (NM) dose and complying with the American Society of Nuclear Cardiology (ASNC) final guidelines effective Jan. 1, 2014. Maine Medical Partners (MMP) – MaineHealth Cardiology in South Portland is one of ten facilities within MaineHealth that is installing the product, with others scheduled to continue roll out before the end of 2013.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
In patients with atrial fibrillation, delayed enhancement magnetic resonance imaging (DE-MRI) performed before ablative treatment can stage the degree of damaged heart tissue (atrial fibrosis) and help predict whether treatment will be successful or not, according to results of Delayed Enhancement — MRI determinant of successful Catheter Ablation of Atrial Fibrillation (DECAAF) trial.
At RSNA 2013, Sony will be showing a works-in-progress medical recorder, the HVO-500MD (without DVD optical drive) and the HVO-550MD (with DVD optical drive). These models are designed specifically for medical recording.
Toshiba is introducing a new Aquilion One platform at RSNA 2013. The new Aquilion One family allows providers to more precisely choose the system that fits their needs today while giving them an upgrade path for the future.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 9, 2013 — Toshiba’s new Aquilion Prime is a versatile, scalable and patient-friendly computed tomography (CT) system. The system’s technologies are built for the clinical and financial needs of users both in the present and future.
September 9, 2013 — Keystone Heart, a company in the development of cerebral protection devices for interventional cardiology and cardiac surgery procedures, announced its TriGuard cerebral protection device has received CE mark, enabling the company to commercially market the TriGuard in Europe and other territories.
The U.S. Food and Drug Administration (FDA) has granted 510(k) market clearance for Siemens’ Symbia Intevo, the first system to completely integrate high sensitivity of single-photon emission computed tomography (SPECT) with the high specificity of computed tomography (CT) into a single modality, rather than imaging as separate overlay images.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
At the 2013 Radiological Society of North America’s annual meeting (RSNA 2013), aycan, a provider of cost-saving PACS solutions, will highlight new release plugins for its post-processing workstation, aycan OsiriX PRO, which significantly expands the functionality of its specialty workstations for mammography, oncology and vascular surgery.
Cardiac stents to open blocked heart arteries and reduce chest pain have been used for decades. However, cardiologists have never been certain that women benefitted from their use because clinical trials testing stents only included, at most, 25 percent women, making the overall findings mostly relevant to just men.
Medtronic Inc. has formed Medtronic Hospital Solutions, a new business focused on developing novel partnerships with hospitals to provide services directly related to hospital operational efficiency. The new business will focus initially on offering services in Europe to manage and modernize catheterization laboratory (cath lab) facilities, bringing sustainable efficiencies and programs to this critical area of hospital cardiology departments.

 September 13, 2013
September 13, 2013