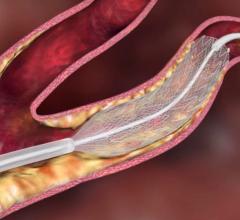
Xact stent by Abbott Vascular
The long-standing standard of care for patients with carotid artery disease was surgery and/or medical management. In August 2004, the U.S. Food and Drug Administration changed all that when it approved the first carotid stenting system, called the RX ACCULINK Carotid Artery Stent System and RX ACCUNET Embolic Protection System by Guidant.
By acquiring the vascular interventions business unit of Guidant earlier this year, Abbott Vascular gained the RX ACCULINK system, adding a second carotid artery stenting (CAS) system to Abbott's CAS offering. In September 2005, approximately a year after Guidant received its FDA approval for a CAS system, Abbott received its clearance for the Xact Carotid Stent and 510(k) clearance for the Emboshield Embolic Protection System.
Currently, Abbott Vascular (formerly Abbott Vascular Devices) is the only medical device company to offer CAS systems. The company believes that the two technologies complement one another and provide better options for the treatment of patients affected by carotid artery disease. Following is a dialog with Mary Bellack, vice president and general manager, Carotid and Neurovascular, Abbott Vascular, who elaborates on the devices.
Diagnostic & Invasive Cardiology (DAIC): What has changed since the approval of carotid stents in the U.S.?
Mary Bellack (MB): CAS has rapidly become a major procedural tool that clinicians are considering along with surgery and medical approaches. At present it would be fair to say that the number of patients overall being treated for occlusive carotid disease is somewhat higher than two years ago, since CAS is approved to a large degree in patients for whom surgery is deemed too risky. These patients fall into the conditions approved by FDA for these devices, whether symptomatic or asymptomatic.
Along with this change in how carotid disease is treated, we are seeing many vascular surgeons — who have traditionally performed carotid endarterectomy surgery (CEA) — learning endovascular stenting as an option for their patients. CMS has also recognized CAS as an alternative to CEA in high-risk patients and now covers CAS in a limited patient group. Further coverage expansion is expected as more evidence accumulates on CAS.
Several trials and/or registries are ongoing in the U.S. to help determine whether use of CAS should be broadened to include patients with less severe occlusions, patients who are not symptomatic or patients who are not high surgical risks. ACT 1 and CREST are the two largest studies of this type.
DAIC: Abbott has been evaluating the effectiveness of CAS in asymptomatic patients. Tell us where things stand with the ACT 1 trial and what other research efforts are underway to evaluate the effectiveness of CAS in both more complex and less severe carotid cases.
MB: CAS is still in its early stages, but there has still been significant evolution of the procedure, as is often the case with new approaches once they become more broadly used. In particular, as physicians are trained and then begin to utilize carotid stenting, they learn a lot about patient selection and how important this is to the success of the procedure. In the very early days of interventional treatment of the carotid arteries, physicians used balloon angioplasty without stents, which resulted in some of the same type of challenges encountered in the early days of balloon angioplasty in the coronary arteries. However, the use of stents, particularly stents specifically designed for the carotid artery, have dramatically reduced such problems.
Given substantial procedure evolution and refinement, CAS is now being evaluated head to head against CEA in “normal risk” patients, the approximately 75 percent of patients who are at only moderate risk for CEA. CREST (normal risk, asymptomatic and symptomatic) and ACT 1 (normal risk, asymptomatic) are the landmark studies that will define the role of CAS versus CEA in mainstream patients.
Interesting research is also being conducted in asymptomatic patients specifically looking at the patient's neurocognitive symptoms pre- and post-CAS. Traditionally, these patients were defined as “asymptomatic” as they had no transient ischemic attack (TIA) or stroke in the previous six months. However, early research is suggesting these patients may have subtle deficits that are picked up by neurocognitive testing. This may shed new light into whether these patients are truly “asymptomatic.”
DAIC: What role, if any, do carotid endarterectomy and medical management have in a CAS era?
MB: Although CAS is growing rapidly and is seen as an important new therapeutic option, it is still in its infancy, with current penetration into CEA estimated at 20 percent worldwide. Mostly, CAS is an option for patients whose medical condition or anatomical factors place them at high risk for complications from CEA. Of course, if CREST/ACT 1 show positive results in the normal risk patient population, this picture will change dramatically.
While CAS is expected to further penetrate CEA over time, medical therapy will remain a mainstay of treatment, in particular for those asymptomatic patients with lower grade carotid stenosis who are at lower risk of stroke. Those asymptomatic patients with more severe stenosis have a higher stroke risk if treated conservatively/medically and do benefit from revascularization, as demonstrated by the landmark ACAS and, more recently, ACST trials, with an approximate 50 percent reduction in stroke at five years.
Given cost and other considerations, there is extensive debate in the clinical community over which specific subset of asymptomatic patients should receive any revascularization — whether CAS or CEA — and which should be treated medically.
In general, the excitement over CAS has probably resulted in greater awareness of the various treatment options and which one best fits the patient's condition.
DAIC: Emboli pose a serious threat to patients undergoing carotid angioplasty. Discuss the role embolic protection has had in safeguarding patients undergoing CAS and angioplasty procedures, and describe Abbott's Emboshield capture device.
MB: Embolic protection devices (EPDs) have been an important step forward in protecting patients from emboli released during interventional treatment of carotid artery disease, which can lead to stroke. Although there is as yet no overwhelming data to show that patients are safer if EPDs are used, most clinicians accept the benefit of using this “safeguard” device. Abbott's Emboshield embolic protection device is an 'umbrella-like' polymer filter that is delivered over a guide wire prior to stent placement to capture any embolic material. Any particles are removed along with the filter after stent placement.
DAIC: Guidant was the first company to receive FDA approval for its carotid stenting system in August 2004. Broad use of CAS was initially inhibited by the fact that only one CAS system (Guidant's) was commercially available. How has approval of Abbott’s Xact and Emboshield changed the market landscape?
MB: There are now two FDA-approved systems for carotid stenting available to U.S. clinicians. Overall, the growth of CAS has been quite rapid.
The availability of CAS was somewhat limited by the rate at which M.D.s could be trained on the Guidant system and then credentialed by their department and hospital. However, many physicians have now been trained and it is the fairly restrictive reimbursement environment that is limiting CAS adoption, even within the high-risk population that is FDA approved.
Also, both Guidant and Abbott set up training programs that have significant requirements a clinician must meet before he or she can be trained on either system. These requirements were developed in extensive cooperation with multiple physician societies and highly experienced individual clinical experts. For each system, the physician had to qualify in order to enter the training session provided by each company.
DAIC: What distinguishes Abbott’s Xact and Emboshield combo from the Guidant (now Abbott Vascular) Acculink and Accunet CAS/EPD system?
MB: These two systems are significantly different from each other, making them complementary rather than directly competitive.
For example, the Xact stent is a closed cell design that results in a stent that is stiffer than the Acculink stent, which provides an excellent result in a certain group of patients with certain anatomies. The more flexible Acculink stent is appropriate for patients with more tortuous anatomy.
Like the stents, the embolic protection devices are also different. The Emboshield operates on a bare wire design, which allows the clinician to cross the lesion with just the wire before the EPD is introduced. The Accunet is built on a fixed wire design, where the EPD is “set” on the guide wire and is inserted as a system. Because these stent/EPD systems are complementary, and because this therapy is still evolving, Abbott will maintain both systems in order to have the optimal product solution for a broader range of patients.
DAIC: Discuss the learning curves associated with percutaneous carotid interventions that may or may not lead to higher complication rates and more adverse events.
MB: So far, the experience with both systems in the hands of a broader range of clinicians versus the small number who participated in the clinical trials is encouraging.
Both Abbott and Guidant are mandated by FDA, as part of their approvals, to enroll a large number of patients treated post-approval in a “real-world” setting. So far, the data seems to indicate that the training programs work well, since the rate of adverse events in this post-approval group is comparable to that of the smaller, clinical trial group.
While intuitively we know that there is a significant learning curve for interventional procedures and, more specifically, for CAS, data from the large CAPTURE post-approval study — which was presented at TCT 2005 and ACC 2006 — showed no meaningful differences in the stroke/death/MI rate [for cases in which] physicians [have] low, medium or high experience levels.
DAIC: Where do things stand with regard to CAS procedure reimbursement?
MB: As of now, reimbursement from CMS/Medicare is rather severely limited, with coverage only for a narrow subset of patients who are FDA indicated. Specifically, CMS covers those patients who are at high risk for surgery and who are symptomatic with >70 percent stenosis. There is an important group of asymptomatic patients who are at high risk for surgery and who also are at high risk for stroke — specifically those asymptomatic patients with severe (>80 percent) stenosis. There have been extensive discussions with CMS about making CAS available to these patients. Some private insurers have shown a willingness to reimburse for patients who fall outside the current CMS guidelines.




 June 26, 2023
June 26, 2023