October 15, 2021 — The InspireMD CGuard Embolic Prevention Stent System (EPS) device for the treatment of carotid artery disease (CAD) and stroke prevention has received reimbursement in France from the National Commission for the Evaluation of Medical Devices and Health Technologies (CNEDIMTS) of the French National Authority for Health (HAS). The CGuard EPS is being added to the list of reimbursed medical products (LPPR) effective Oct. 25, 2021.
This was the final step to full commercial launch of CGuard EPS following CNEDIMTS’ positive opinion for reimbursement received by the company on May 11, 2021, for the treatment of symptomatic and non-symptomatic lesions when surgery is not indicated.
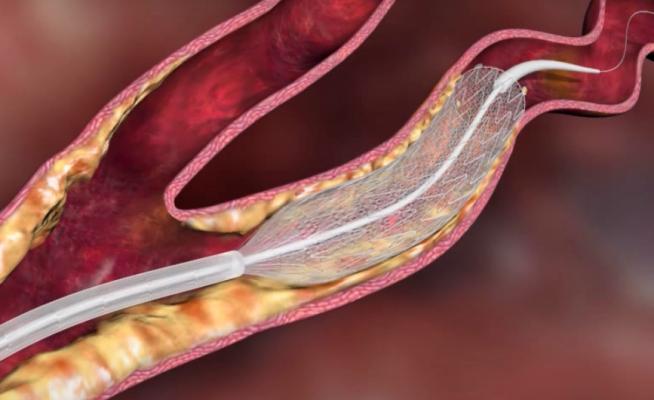
The CGuard EPS Self-Expanding Carotid Stent is the latest generation open-cell nitinol self-expanding stent with patented MicroNet mesh technology designed to prevent the risk of early and late embolism.
“This milestone now provides physicians in France with the choice to use CGuard EPS in the treatment of carotid artery disease and stroke prevention. We strive to improve the standard of care in the treatment of carotid artery disease, by moving away from surgical endarterectomy towards less invasive options such as the CGuard EPS Carotid Stent System. We believe that the unique and proprietary design of our system, is the most advanced and safest stent system on the market today,” said Marvin Slosman, CEO of InspireMD.
The CGuard carotid stent is commercially established in 33 markets.
The device is currently undergoing the C-Guardians U.S. pivotal trial to seek FDA clearance (ClinicalTrials.gov Identifier: NCT04900844).
For more information: www.inspiremd.com


 November 24, 2025
November 24, 2025 









