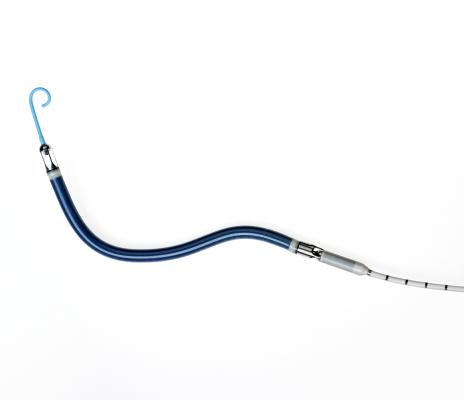
January 28, 2014 — Abiomed Inc. said the Impella RP (Right Percutaneous) system has received U.S. Food and Drug Administration (FDA) approval under a humanitarian device exemption (HDE). This is the first percutaneous single access heart pump designed for right heart support to receive FDA approval. Abiomed completed the HDE submission for the Impella RP in September 2014 following the completion of the RECOVER RIGHT study.
Delivered through a catheter requiring only a small hole in the leg, the Impella RP is FDA indicated for providing circulatory assistance for up to 14 days in pediatric or adult patients who develop acute right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. These patients lack blood flow from the right side of their hearts. The Impella RP is designed to provide the flow and pressure needed to compensate for right heart failure. The device does not require a surgical procedure for insertion, and it provides up to 4 liters per minute of hemodynamic support.
“The Impella RP represents a huge step forward in offering right side support using a minimally invasive platform and has the potential to transform interventional cardiology and cardiac surgery today. With the ability to place this device percutaneously on the right side, physicians can now treat acute right sided heart failure minimally invasively and quickly,” said Mark Anderson, M.D., co-principal investigator for the RECOVER RIGHT trial and chair of the division of cardiothoracic surgery at Einstein Medical Center.
There will be a controlled launch of the Impella RP in the United States after each site completes in-house training at Abiomed. This rigorous training will incorporate members of the heart team, including the interventional cardiologist, cardiac surgeon, heart failure cardiologist and lead nurse.
As part of the HDE approval, Abiomed is required to conduct two post approval studies (PAS) for the RECOVER RIGHT. One includes an adult patient population of 30 patients and the other, a pediatric patient population for a maximum of 15 patients (larger patients < 18 years of age with RVF.) These studies will be conducted to monitor the post-market safety and probable benefit of the Impella RP device. Both studies will be a single-arm multicenter studies that will follow the respective patients at 30 and 180 days post device explant.
RECOVER RIGHT Study Results
RECOVER RIGHT was an FDA-approved, prospective, multicenter, single arm study designed to evaluate the safety and probable benefit of the Impella RP in patients with right ventricular failure (RVF) refractory to medical treatment and deemed to require hemodynamic support.
The 30 patients enrolled in the RECOVER RIGHT trial were categorized into two patient cohorts. Cohort A included patients who developed RVF within 48 hours after implantation of a left ventricular assist device (LVAD). Cohort B examined patients who developed RVF within 48 hours of post-cardiotomy shock or post-acute myocardial infarction (AMI) shock. The primary endpoint was patient survival at 30 days, hospital discharge, or bridge to the next therapy.
The clinical trial results from RECOVER RIGHT were announced in October 2014 at the annual Transcatheter Cardiovascular Therapeutics (TCT) 2014 scientific meeting in Washington, D.C. Overall, the survival rate was 73 percent in the entire population at 30 days. Cohort A showed a survival rate of 83.3 percent and Cohort B showed a 58.3 percent survival rate at 30 days.
In May 2014, Abiomed received approval for a Continuous Access Protocol (CAP) from the FDA for RECOVER RIGHT.
For more information: www.abiomed.com

