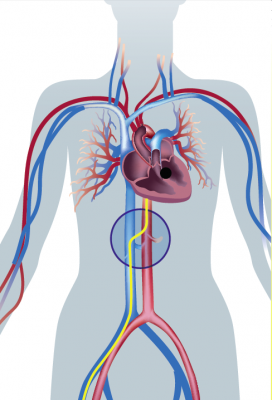
December 23, 2014 — For patients transcatheter aortic valve replacement (TAVR) patients whose anatomy prevents the use of femoral artery access, use of a transcaval access route (accessing the femoral vein and then tunneling into the aorta) may offer a new access route option.
Adam Greenbaum, M.D., co-director of the Henry Ford Center for Structural Heart Disease, helped perform the first successful transcaval access route to perform TAVR in Europe Dec. 1, sharing his expertise on a pioneering way to access the heart. Henry Ford was the first hospital in the world to perform the unique procedure, which accesses the heart for valve replacement by temporarily connecting major blood vessels in the abdomen. Greenbaum, who taught the technique to seven other centers in the United States, was asked to share the technique at the German Heart Centre Munich with Markus Kasel, M.D. They performed the technique for a middle-aged German man, who is recovering nicely, Greenbaum reported.
Watch a 2017 interview with Greenbaum in the VIDEO: Transcaval Access in TAVR Procedures.
"We are pleased that the advanced cardiology procedures we perform at Henry Ford can help patients both here and around the world," said Greenbaum. Henry Ford cardiologists estimate the procedure could help 25,000 to 50,000 patients in the United States annually, when scar tissue, small arteries or other medical issues prevent cardiologists from using traditional access to the heart. Now, the option will be available to patients in Europe, too, Greenbaum said.
Henry Ford Hospital cardiologists performed the health system's first transcaval procedure on July 3, 2013, in Detroit. Traditional valve replacement was not medically viable for an 80-year-old northern Michigan great-grandmother, who's alive and well today.
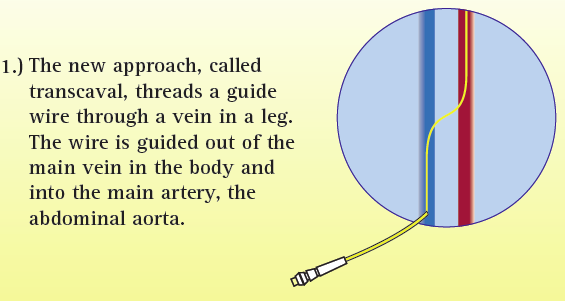
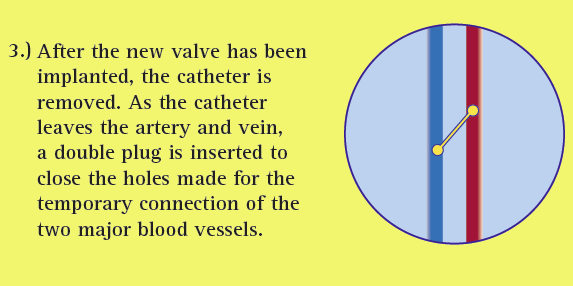
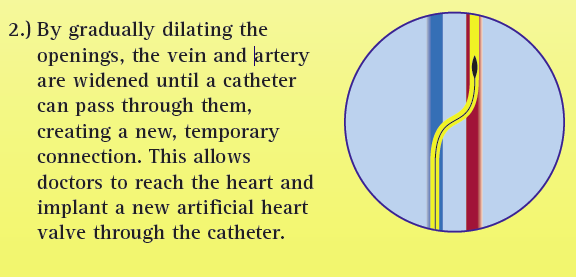
During transcaval valve replacement, a wire is guided into a leg and up through the femoral vein into the inferior vena cava (IVC). The IVC is punctured with a 0.014-inch guidewire, which has been connected to a cautery device to provide energy to the tip of the guidewire. The access point is dilated with the dilator that comes with the valve delivery sheath. The delivery sheath for the TAVR valve goes across the IVC into the infrarenal aorta. The puncture site is closed by using a St. Jude-AGA Medical VSD occluder, 6- or 8-mm in diameter, Greenbaum explained
"Angiography alone is used to guide access and closure. We use a contrast CT of the aorta before the procedure to assess suitability and choose the level at which to cross into the infrarenal aorta," he said. "We make sure no structures like bowel loop or major side branches are at the level of crossing by examining the CT before the case. Typically, the access is at the level of the L3-L4 disc space, which we use for geographic landmarks."
The technique was perfected by Greenbaum, Henry Ford cardiologist William O'Neill, M.D., and Robert Lederman, M.D., an interventional cardiologist at the National Heart, Lung, and Blood Institute, who developed the transcaval technique in a research setting. Greenbaum led the team who performed the first procedure at Henry Ford. He worked alongside O'Neill and Gaetano Paone, M.D., division head of cardiac surgery at Henry Ford Hospital.
For more information: www.henryfordhospital.com/structuralheart


 December 24, 2025
December 24, 2025 









