
The iFR modality provides a hyperemia-free measurement in as few as five heartbeats.
Earlier this year, the Barnes-Jewish Hospital at Washington University School of Medicine in St. Louis adopted the instant wave-Free Ratio (iFR) technology. The iFR modality is a pressure-derived hyperemia-free index for assessment of coronary stenosis relevance.
Early indications suggest significant economic and patient care advantages may exist with the adoption of iFR. Specifically, procedural time and costs are reduced vis-à-vis current physiological assessment procedures and the patient experience is enhanced. In my experience, risks of inappropriate procedures and potential readmits are reduced with this new technology. Furthermore, by removing barriers to use, with iFR clinicians may actually be able to do more appropriate procedures by using physiology to guide their treatment decision. Last but not least, patient satisfaction is no longer an intangible benefit as the Affordable Care Act has tied reimbursement to satisfaction.
FFR as Predecessor
Fractional flow reserve (FFR), the precursor technology to iFR, has done well to prove the patient and economic benefits of physiology over the static “see and stent” modality of the past. FFR, like iFR, is a physiological assessment that determines if a lesion or other stenosis actually results in limiting blood flow and by how much. Despite FFR’s remarkable success, adoption remains very low in many parts of the world. There remain improvements necessary to achieve the additional benefits the technology promises.
Complaints about FFR include the extra time it takes to set up and perform measurement and the use of adenosine to induce stress. The drug can add cost to the procedure and cause discomfort for the patient.
Underlying Concept of iFR
Since blood flow is difficult to measure directly, we measure it indirectly by measuring pressure. This convention holds if resistance remains constant — which we know it does not over the whole cardiac cycle. However, there is a short period during diastole where resistance is relatively constant — hence the “wave-free” characteristic of iFR and what sets it apart from FFR. With the help of very sophisticated and automated algorithms, the translesional pressures are taken precisely when resistance is stable and minimal, and accordingly, changes in pressure are directly relatable to changes in blood flow.
iFR in Practice
Like many physicians, I was skeptical in the beginning, but after getting a better understanding of the physiology of iFR, I decided to implement it in my practice at Washington University School of Medicine.
Incorporating iFR into the cath lab was relatively simple. It is a software upgrade to existing Volcano equipment. To get a baseline measurement, a pressure wire — the same one as used for FFR — is inserted. There is a toggle switch that you can move easily from iFR to FFR within the same case, but it is usually left in the iFR mode. Once the wire is in, it is normalized then passed down the lesion and across the stenosis. At this point, a button is pressed to calculate iFR values and record. In our practice, we typically take three readings to ensure stable, consistent results.
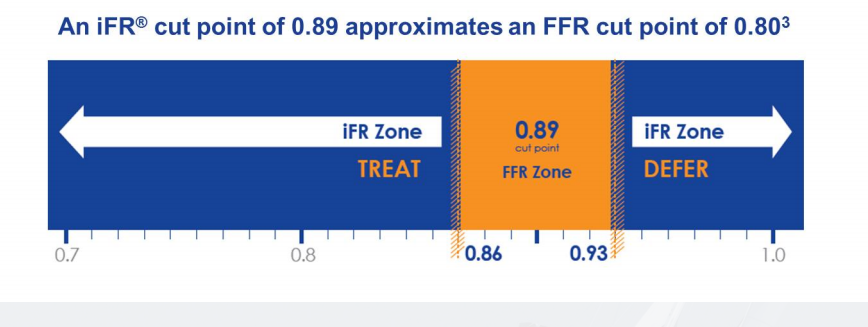
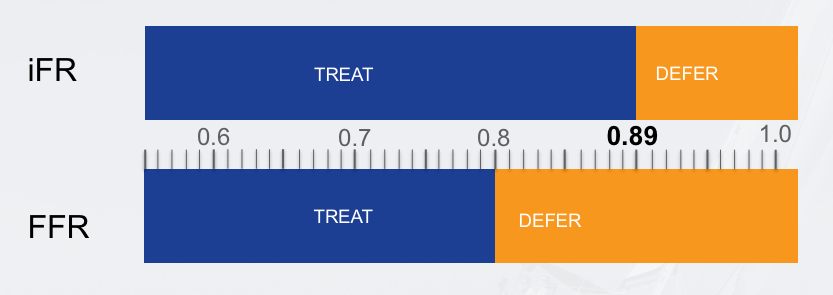
At Barnes-Jewish Hospital we have been executing a dual approach in order to build up our registry data and also to add to the significant and growing empirical evidence for use of iFR and its correlation to FFR. We initially perform iFR on everyone who needs physiologic assessment, and then also perform FFR on those same patients. If not for the registry, we would be using a hybrid approach as follows: if the iFR value is in the “gray zone” (between 0.86 and 0.93), then you administer adenosine and do FFR. If the value is less than 0.86, this is indicative of the presence of ischemia and a stent is inserted and the results are re-checked post-intervention. If the iFR value is greater than 0.93, this is indicative of the absence of severe ischemia and deferral in favor of optimal medical management is warranted.
Barnes-Jewish Hospital has completed more than 100 cases and holds the largest user registry in the United States on iFR. Our findings to date correlate with the findings overseas and with the original studies. Once outcome studies like DEFINE Flair (NCT02053038) can demonstrate that it is safe to defer patients with a cutoff of, say, 0.89, we will move toward relying more on iFR only. Robust and evidentiary data are just not available currently, so that is why FFR is being used clinically within the gray zone.
Economic Benefits
A nascent technology, iFR is clearly tracking toward substantial benefits accruing to patients, caregivers and hospitals. Through technology enhancements, reduction in adenosine use and substantial time savings (against FFR baselines), iFR saves an additional $250—$400 per procedure on top of the documented cost savings in the FAME Trial data of $675 per procedure using FFR.
Right now iFR has obviated the need to administer adenosine in approximately 65 percent of our cases. As studies complete, this number will go higher. A conservative time savings estimate (mixing the drug, patient setup, the time required to obtain central venous access and the time for infusion) is 15 minutes per procedure, thus increasing the turnover time of the cath lab.
Patient satisfaction is an increasingly important measurement in assessing quality of care and is a key determinant in pay-for-performance metrics. Under the Centers for Medicare and Medicaid Services (CMS) Hospital Inpatient Value-Based Purchasing (HIVBP) program, Medicare reimbursements are linked to patient satisfaction and surveys completed by patients. We believe it is reasonable that patient satisfaction will increase as iFR use grows. They will not experience the adenosine side effects associated with FFR such as tightening of the chest, feeling flushed or anxious, or even in some cases feeling like they may die. Furthermore, some patients experience increased risk due to adenosine, especially those with asthma or abnormal hearts.
With the potential for more physiology-guided percutaneous coronary interventions (PCIs), costly readmits may decline. Current data show we use fewer stents with the FFR procedure, which equates to significant cost savings. And fewer stents result in fewer complications and readmits.
The utility of physiology-guided PCI is not limited to only patients who will either be treated with PCI or deferred. One of the challenges still facing interventionalists today is the patient presenting with multi-vessel disease. Contemporary evidence suggests, when using an angiogram alone, these patients should be referred to surgery. But as we learned with the FAME studies, and will learn further with the now-enrolling SYNTAX II study, not all of the lesions we see with our eyes are creating ischemia for the patient. With easier to use physiologic measures like iFR, we will lower the burden of doing multi-vessel coronary physiology in these complex patients. This will most likely enable clinicians to increase the total number of stents by treating more difficult cases in the cath lab instead of referring them to surgery.
Reimbursement
Reimbursement is a critical component of the economic benefits of iFR. As of August 2014, the American College of Cardiology (ACC) and the Society for Cardiac Angiography and Interventions (SCAI) are now recommending iFR measurements be coded using the FFR CPT codes with a -52 modifier. With reimbursement, physicians will be more inclined to use the procedure and when they experience how easy it is, how much better the patient experience is and how they can save costs, adoption will increase rapidly. From the hospital perspective, it will verify appropriate treatment, improve patient care and save costs immediately and down the road.
Impediments to Adoption
One impediment to adoption we are seeing revolves around culture change. Many physicians are uncomfortable with iFR initially because they are skeptical of resting readings. Most doctors were taught in medical school that you measure at rest and then you exercise and do a stress test. This is what adenosine simulates in FFR. Using a resting measurement (iFR) to decide whether a lesion is significant runs counter to the ingrained adage that hyperemia or stress is necessary to properly assess lesions. Another obstacle impeding iFR concerns a lack of understanding surrounding physiology assessments in general and the fact that we as interventionalists have been trained on treatment decisions based on the angiogram. We believe these related impediments can be overcome through more data and greater education.
Summary
I am confident the many outcome studies ongoing and planned will conclude incontrovertible evidence for increased reliance on iFR. And with that increased use of physiologic-guided procedures comes increased economic benefits and cost savings, as well as a decline in inappropriate procedures. I truly believe that physicians want to do the right thing, but sometimes they do not out of convenience, or because that is the way they have been doing it. So with its ease of use and reduced costs, iFR will help lead the way to more appropriate procedures and enhanced patient care.
Editor’s note: Jasvindar Singh, M.D., is assistant professor of medicine and director of the cardiac cath lab at Washington University School of Medicine in St. Louis, Mo. Growing up in Fiji, he became an accomplished chess player, earning international ratings representing his country in three Olympiads in 1986, 1988 and 1990.




 November 24, 2025
November 24, 2025 









