
The Genous R-Stent uses a prohealing surface utilizing endothelial progenitor cell (EPC) capture technology to aid rapid stent endothelialization. Photo copyright OrbusNeich
In the world of endovascular devices, the rule is no longer one size fits all. The recent increase in the variety of stents for percutaneous coronary intervention (PCI) offers the physician of a patient with coronary artery disease (CAD) the ability to tailor treatment to the individual patient, rather than tailor the patient to the therapy. While bare metal stents (BMS) and drug-eluting stents (DES) are appropriate for many patients, advances in the area of prohealing stent technology have expanded the options for more challenging patient subsets. Having more choices may potentially lead to better patient outcomes.
BMS and DES were originally studied in strictly controlled trial populations that generally excluded more challenging patient subsets. Therefore, these study populations do not effectively reflect the real world of CAD that includes patients with, or at high risk of, bleeding disorders, elderly patients, other co-morbid factors, such as diabetes or planned noncardiac surgery in the near term.
The Need for Antiplatelet Therapy
For patients treated with DES, the American College of Cardiology (ACC) and American Heart Association (AHA) treatment guidelines recommend at least 12 months of dual antiplatelet therapy (DAPT) with aspirin and clopidogrel after percutaneous coronary intervention (PCI). Stenting initially exposes a metallic “foreign body” to constituent circulating blood cells, increasing the risk for thrombosis. Additionally, the metallic stent and, in the case of first generation DES, the nondegradable polymer can induce inflammation that leads to further, potential adverse cardiac events. Blood-thinning DAPT reduces the risk of thrombosis during the healing process and can be discontinued once the stent has endothelialized. In many cases, for example, DAPT therapy is prolonged following DES implantation when the risk of thrombosis at the site of stent placement remains high, because of delayed or incomplete re-endothelialization.
While the risk-benefit profile of this standard of care is satisfactory in the majority of cases, for patients who have a higher risk of bleeding, an abbreviated duration of DAPT would be beneficial. In addition, in elderly patients the one-year requirement of DAPT is generally undesirable and can lead to bleeding problems. Equally, in patients with CAD, 5 percent require noncardiac surgery within 12 months following PCI. Surgery in the near term can increase the risk of stent thrombosis due to the increase in platelet volume and DAPT can increase the risk of bleeding. As a result, these patients are in the precarious position of requiring the premature stop of blood thinning agents in a prothrombotic state.
Promoting Endothelialization
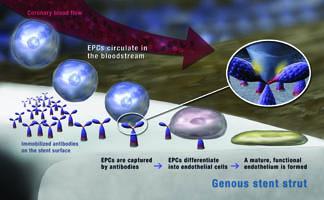
Advancement in the design of prohealing surfaces have led to stents that can reduce the required duration of DAPT by decreasing the time required for stent endothelialization, while still maintaining the patency of the coronary artery. One such prohealing surface utilizes endothelial progenitor cell (EPC) capture technology. The surface consists of a matrix containing monoclonal antibodies that target a receptor unique to EPCs circulating in the blood stream. EPC capture promotes their maturation to endothelial cells and accelerates the body’s tendency for natural endothelialization.
The Genous Bioengineered R Stent developed by OrbusNeich combines EPC capture technology with a modern structural stent design to accelerate healing of the vessel wall. With the risk of thrombosis decreasing rapidly as the vessel heals, patients treated with the Genous stent are usually only prescribed four weeks of DAPT.
The Genous Bioengineered R Stent is an example of an option for treating CAD that increases the physician’s flexibility. While not used in the majority of my PCI procedures, I have used more than 200 Genous stents with good results in selected patients. In my opinion, the benefit of the newer prohealing surfaces on stents is an improved balance between safety and efficacy in such selected patients.
Reducing Antiplatelet Therapy
The Genous stent is able to achieve the necessary patency of the coronary artery over the long-term without the price of prolonged DAPT. Data from the e-HEALING prospective clinical registry for the Genous stent have confirmed safety and efficacy of the stent in more than 1,500 elderly patients.
In my experience, a subset of patients who benefit from a short duration of DAPT with the Genous are those with lung cancer, for whom immediate surgery to remove the tumor is both nondeferrable and potentially curative. Some of these patients have also been diagnosed with coexisting CAD, which requires treatment before their urgent surgery. Their course of blood thinners can be shortened to only two weeks with the Genous stent and following this they can subsequently undergo the potentially life-saving surgery their cancer diagnosis requires.
Formerly, the approach to cardiac intervention was very mechanical — get in, expand, get out. Now, with the new compelling advances in biomechanical stent design, there is an increased focus on the healing properties of endovascular devices for PCI and a desire to improve vessel healing post stent procedure. The ultimate goal of PCI is long-term arterial patency. While prolonged DAPT is not ideal, for many patients the risk-benefit profile of DES and 12 months of DAPT is satisfactory. However, for the challenging real-world patient subsets rarely studied in clinical trial populations, in whom prolonged DAPT is not desirable, stents that reduce this requirement offer more choice to the treating physician. PCI can now be tailored to the individual’s unique medical profile. Stent technology continues to progress with others building upon the notion of better vessel healing by combining components of DES technology with other antibodies and biologics to promote better vessel healing.
Editor’s Note: Dave Smith is a consultant cardiologist at Morriston Hospital, Swansea, U.K., specializing in interventional cardiology. His clinical interests include management of the spectrum of coronary artery disease from chronic stable angina to acute myocardial infarction. He specializes in radial access angioplasty, intravascular ultrasound (IVUS) and percutaneous aortic valve replacement.
References:
1. Luckie M., Khattar R. S. and Fraser D. “Noncardiac Surgery and Antiplatelet Therapy Following Coronary Artery Stenting.” Heart. Feb. 21, 2009
2. Klomp M., Beijk M. A. and de Winter R. J. “Genous Endothelial Progenitor Cell-Capturing Stent System: A Novel Stent Technology.” Expert Review of Medical Devices. July 2009. 6 (4) pages 365-375.
3. Piscione F., Cassese S., Galasso G., Cirillo P., Esposito G., Rapacciuolo A., Leosco D., Piccolo R., De Rosa R. and Chiariello M. “A New Approach to Percutaneous Coronary Revascularization in Patients Requiring Undeferrable Noncardiac Surgery.” International Journal of Cardiology. Aug. 21, 2009.
4. Iniguez. “OrbusNeich’s Genous Bioengineered R Stent Demonstrates Excellent Safety and Clinical Outcomes in High-Risk Elderly Patients.” Presented at Transcatheter Cardiovasculkar Theraputics Asia/Pacific(TCTAP) 2010, Seoul, Korea.


 November 24, 2025
November 24, 2025 









