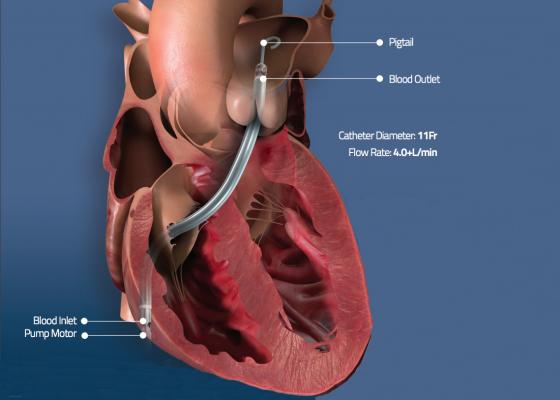
Illustration showing the venous implantation route and where the inlet and outflow ports are when the Impella RP is placed in the right side of the heart.
Right heart failure (RHF) is a syndrome characterized by the inability of the right ventricle (RV) to support optimal circulation and may result in cardiogenic shock. The etiologies of RHF are diverse including acute myocardial infarction (MI), post-surgery or heart transplant, and left ventricular assist device (LVAD) implantation.[1-3] Regardless of the underlying cause, severe RHF is associated with high morbidity, mortality, longer hospital length of stay and higher costs of care.[4]
Under normal conditions, RV function depends on preload (systemic venous return), afterload (pulmonary artery resistance), pericardial compliance and native contractility of the RV free wall and interventricular septum.[2] Thus, alterations such as increased RV afterload, decreased RV preload or contractility result in RV dysfunction (RVD) and treatment strategies are aimed at optimizing the RV preload, afterload and contractility.
Given that right ventricular failure (RVF) is a hemodynamic problem, assessment of hemodynamic parameters including central venous pressure (CVP), cardiac output or index, using the pulmonary artery (PA) catheter in combination with cardiac echocardiography represents the gold standard for evaluation of RV function. Several hemodynamic variables have been used to assess RV function. The simplest approach is to measure cardiac filling pressures based on the ratio of CVP to pulmonary capillary wedge pressure (CVP/PCWP).[2] The normal value for CVP/PCWP is 0.5 and a ratio higher than 0.63 indicates right ventricular dysfunction (RVD). The PA pulsatility index (PAPi), defined as the ratio of PA pulse pressure to CVP, is another marker of RVD. Studies suggest PAPi < 1.0 is a highly sensitive indicator of RV dysfunction in the setting of acute myocardial infarction. However, it is important to note that the thresholds of the CVP/PCWP, PAPi, and other hemodynamic parameters associated with RVD vary based on the underlying etiologies of RVF.
The American Society of Echocardiography (ASE) suggests quantitative assessment of RV function by at least one of the following parameters: tricuspid annular plane systolic excursion (TAPSE, normal reference limit ≥ 18 mm), RV fractional area change (RVFAC, normal value > 35%), or RV tissue Doppler S velocity (normal value > 10 cm/s) of the tricuspid annulus.[1,3] Three-dimensional echocardiography represents an accurate method to assess RV size, contractility and ejection fraction.[1]
The percutaneous mechanical circulatory support device, Impella RP (Abiomed, Danvers, Mass.), provides the opportunity for early intervention in the downward spiral of medically refractory RVF. Given the reversible nature of right RVD, timely initiation of mechanical support may serve as a bridge to recovery, or heart transplant or destination therapy. The Impella RP is a 22 French (F), 3-D catheter-based micro-axial pump approved by the U.S Food and Drug Administration (FDA) for use in acute RHF for up to 14 days.[5] The catheter pump is advanced antegrade under fluoroscopic guidance over a 0.025-inch platinum super-stiff wire and positioned across the tricuspid and pulmonary valves through a 23 F peel-away sheath inserted in the femoral vein. The pump inflow is positioned in the inferior vena cava (IVC) and the outflow in the pulmonary artery (PA); thus, the pump aspirates blood from the IVC and expels it into the PA at a rate of up to 4 L/min. It is recommended to ensure that the tip of the Impella RP is positioned in the main pulmonary artery directed toward the left PA, while the PA catheter is positioned in the right PA in order to monitor RV function accurately.[5]
Clinical Evidence for Right Heart Support
The hemodynamic effectiveness of the Impella RP was first demonstrated in the RECOVER RIGHT study including 30 patients.[4] The study population included 18 patients developing RVF within 48 hours after LVAD implantation and 12 patients developing RVF within 48 hours of cardiac surgery or MI. Initiation of Impella RP support resulted in an immediate improvement in the cardiac index with a concomitant reduction in central venous pressure, translating to improved outcomes with a 30-day survival rate of 73.3%. The clinical benefit of Impella RP was further confirmed in another Impella RP pre-market clinical study of 60 patients with RVF.[6]
Issues With Patient Selection in Post-market Study
However, the recent post-approval study (PAS) involving 42 patients with RVF showed a 30-day survival of 64% in patients who met the RECOVER RIGHT inclusion criteria and only 11% among patients in whom Impella RP was used as salvage support.[7] The poor outcome with Impella RP for salvage patients (as defined by the FDA) demonstrates the need for proper patient selection and early initiation of hemodynamic support with Impella RP.
On the basis of best practices identified in multiple clinical studies and in accordance with the inclusion and exclusion criteria of RECOVER RIGHT, Abiomed has provided a new patient checklist. This checklist helps determine if Impella RP is an appropriate treatment option for the patient or if the patient is not likely to benefit from Impella RP support.
As the saying goes, “the first step is always the hardest,” early identification of RVD and appropriate patient selection for Impella RP support matters most to obtain the best clinical outcomes. In addition, frequent monitoring of RV function using echocardiography and PA catheter measurements is crucial to guide therapy. The spectrum of early identification, early therapeutic intervention, and early escalation remains the current model for success in the management of RHF and cardiogenic shock.
Read the related article "Higher Mortality With Impella RP May be Due to Poor Patient Selection."
Read the October 2019 article "FDA Post Clearance Study Shows Timely Diagnosis of Right Heart Failure and Early Impella RP Use Leads to Better Survival."
Editor's Note: Perwaiz Meraj, M.D. FACC, is director of cardiology research at Northwell, Northshore University Hospital, Long Island, N.Y. He also is an assistant professor at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell. Uma Chandrasekaran, Ph.D., is a medical affairs scientist at Abiomed.
Related Content Involving Doctor Meraj:
VIDEO: Complex PCI Involving Prior CABG and Comorbidities — Interview with Perwaiz Meraj, M.D.
First Patient Enrolled in Study Examining STEMI Door to LV Unloading Time
References:
1. Konstam MA, Kiernan MS, Bernstein D, et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e578–e622. doi: 10.1161/CIR.0000000000000560.
2. Kapur NK, Esposito ML, Bader Y, Morine KJ, Kiernan MS, Pham DT, Burkhoff D. Mechanical circulatory support devices for acute right ventricular failure. Circulation. 2017; 136:314–326. doi: 10.1161/CIRCULATIONAHA.116.025290.
3. Harjola VP, Mebazaa A, Čelutkienė J, al. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016;18(3):226-41. doi: 10.1002/ejhf.478.
4. Anderson MB, Goldstein J, Milano C, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: the prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015; 34:1549–1560. doi: 10.1016/j.healun.2015.08.018.
5. Pieri M, Pappalardo F. Impella RP in the Treatment of Right Ventricular Failure: What We Know and Where We Go. J Cardiothorac Vasc Anesth. 2018;32(5):2339-2343. doi: 10.1053/j.jvca.2018.06.007.
6. Anderson M, Morris DL, Tang D, et al. Outcomes of patients with right ventricular failure requiring short-term hemodynamic support with the Impella RP device. J Heart Lung Transplant. 2018;37(12):1448-1458. doi: 10.1016/j.healun.2018.08.001.
7. Abiomed Press Releases. Impella RP Post-Approval Study Data Presented at ACC 2019. http://investors.abiomed.com/news-releases/news-release-details/impella-rp-post-approval-study-data-presented-acc-2019. Accessed March 19, 2019.





 November 14, 2025
November 14, 2025 









