Heart Rhythm Society (HRS) President-Elect Hugh Calkins, M.D., FACC, director, cardiac arrhythmia services and EP lab ...
Cardiac Resynchronization Therapy Devices (CRT)
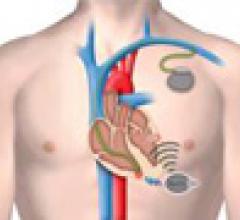
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
May 14, 2012 ? Boston Scientific Corp. announced extended warranties of up to 10 years, depending on the model, for its ...
May 12, 2012 — Sorin Group announced at the Heart Rhythm Society (HRS) annual scientific sessions, the publication of ...
May 11, 2012 — The Sorin Group is launching new implantable devices with a proprietary algorithm system as well as three ...
May 11, 2012 — St. Jude Medical Inc. announced U.S. Food and Drug Administration (FDA) approval of its Assura portfolio ...
May 9, 2012 — Boston Scientific Corp. announced U.S. Food and Drug Administration (FDA) approval and market launch of ...
April 12, 2012 – The U.S. Food and Drug Administration (FDA) this week approved an expanded indication for Medtronic’s ...
April 6, 2012 — St. Jude Medical announced it is proactively informing physicians about visual observations of ...
January 31, 2012 — Medtronic Inc. announced U.S. Food and Drug Administration (FDA) approval and launch of the DF4 High ...
January 26, 2012 — St. Jude Medical recently announced U.S. Food and Drug Administration (FDA) approval of its Unify ...
December 29, 2011 – Heart failure remains by far the single biggest reason for acute hospital admission but is ...
November 18, 2011 – MedSolutions, a provider of medical cost management services, announced the launch of its ...
November 16, 2011 — MedSolutions announced the launch of its Implantable Cardioverter Defibrillator (ICD) Surgery ...
November 16, 2011 – Cambridge Consultants unveiled how it has collaborated with start-up company EBR Systems to develop ...
October 18, 2011 — A U.S. Food and Drug Administration (FDA) committee will discuss and make recommendations to expand ...


 May 17, 2012
May 17, 2012