June 25, 2009 – Today, St. Jude Medical Inc. and Medtronic Inc. both commended the efforts of investigators in the ...
Cardiac Resynchronization Therapy Devices (CRT)
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
June 23, 2009 – Boston Scientific Corp. and the University of Rochester Medical Center announced today that the ...
June 23, 2009 - Today, Medtronic Inc. announced the first worldwide enrollments in the MORE-CARE (Monitoring ...
May 26, 2009 – Biotronik Inc. recently launched its Lumax 540 series implantable cardioverter defibrillators ...
May 18, 2009 - With electrophysiology procedures continuing to grow in a time of increasing economic constraints ...
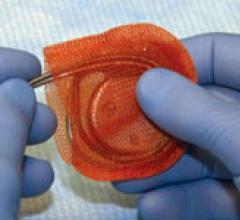
May 18. 2009 - TYRX Inc. announced the initial, interim clinical results from its AIGISRX Antibacterial Envelope ...
May 14, 2009 - St. Jude Medical Inc. yesterday announced European CE mark approval of its Accent RF pacemaker and ...
May 6, 2009 - Medtronic Inc. today said the FDA approval and availability of the Attain Ability left?heart lead ...
April 14, 2009 - Biotronik Inc. announced yesterday the first U.S. implant of the next-generation Lumax 540 Series ...
April 3, 2009 - BIOTRONIK GmbH & Co Kg this week announced the global launch of BIOTRONIK Home Monitoring with ...
April 2, 2009 - The 24-month findings from the REVERSE (Resynchronization Reverse Remodeling in Systolic Left ...
March 19, 2009 – The enrollment phase of the RAFT clinical trial (Resynchronization/Defibrillation for Ambulatory ...

With the healthcare industry striving for greater efficiency at virtually every level, it shouldn’t be a surprise ...
February 6, 2009 – The FDA has approved St. Jude Medical Inc.’s medical device system that allows a single ...
December 22, 2008 - ZOLL Medical Corp. recently said more than 12,000 patients at high risk of sudden cardiac arrest ...

 June 25, 2009
June 25, 2009