July 8, 2010 – Boston Scientific Corp. today announced enrollment of the first patient in its MultiSENSE clinical ...
Cardiac Resynchronization Therapy Devices (CRT)
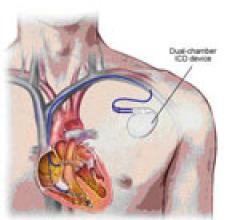
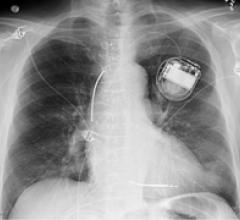
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
June 21, 2010 – MADIT-CRT trial data shows women receive greater clinical benefit from cardiac resynchronization ...
June 17, 2010 – The Protecta portfolio of defibrillators will soon be available in Europe. Medtronic Inc. today ...
June 1, 2010 – A quadripolar pacing system was recently released in Europe and offers physicians the ability to ...
May 14, 2010 – New evidence demonstrates key shock-reduction programming strategies significantly reduced ...
May 13, 2010 – An integrated, break-away hemostasis valve is integrated into a new lead delivery system is ...
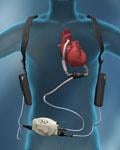
New U.S. Food and Drug Administration (FDA) indications for two key heart failure device therapies may help ...
Monitoring congestive heart failure patients for any worsening in their condition is based mainly on patient ...
May 11, 2010 – A cellular accessory has been added to an implantable cardiac device remote monitoring system to ...
May 11, 2010 – A wireless USB adaptor allows patients with implantable cardiac devices to stay connected with ...
May 4, 2010 — New research released this week in the Journal of the American College of Cardiology shows the value ...
March 24, 2010 – Enrollment began this week in the CITADEL, which is the second of two large-scale, prospective ...
March 22, 2010 – The FDA’s Circulatory System Devices Panel last week unanimously recommended expanding the ...
February 8, 2010 – The FDA cleared the Sorin Paradym CRT Model 8750, a cardiac resynchronization therapy ...
February 5, 2010 – European CE mark approval was granted and first implants reported for the Fortify and Fortify ...

 July 08, 2010
July 08, 2010