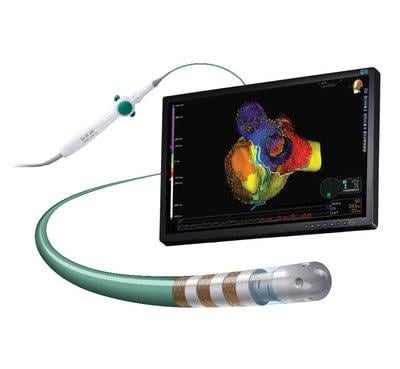
May 10, 2017 — Abbott announced CE Mark of the TactiCath Contact Force Ablation Catheter, Sensor Enabled, developed to make it easier for physicians to more effectively treat atrial fibrillation (AF). When integrated with Abbott's EnSite Precision cardiac mapping system, physicians are able to utilize dual impedance and magnetic technologies to help more precisely model the heart. This integrated system also helps physicians determine where to apply optimal contact force (pressure) when creating a lesion during a cardiac ablation to correct a heart rhythm abnormality. The Sensor Enabled technology allows physicians to create a more detailed heart model during ablation procedures than a catheter without a sensor.
Arrhythmias develop when electrical signals that regulate heart rhythms become disrupted or change, making the heart develop an irregular heartbeat. A patient with AF is at increased risk for stroke because the rapid heartbeat can let blood pool in the heart, which can cause clots to form and travel to the brain. To restore optimal electrical signals in the heart, ablation catheters deliver radio-frequency energy to cardiac tissue, creating small scars or lesions to areas responsible for generating arrhythmias. Not all ablation technology is the same, however, and recent research has continued to show the importance of applying optimal contact force to the heart during ablation procedures. Too much pressure applied to heart tissue can result in complications, yet if not enough pressure is applied the lesion may not be effective enough to stop the erratic signals in the heart.
"The TactiCath Contact Force Ablation Catheter, Sensor Enabled along with the EnSite Precision cardiac mapping system create a powerful combination for more precisely treating patients with cardiac arrhythmias," said Martin Lowe, M.D., clinical director of cardiac electrophysiology at Bart's Heart Centre at St. Bartholomew's Hospital in London. "Feeling confident in the accuracy of the contact force reading allows me to target the optimal pressure for creating effective and safe lesions."
The device is an update to the TactiCath Contact Force Catheter family. The clinical evidence show use of optimal Contact Force-guided AF ablation with the TactiCath Quartz catheter resulted in clinical success in 85.5 percent of patients and fewer post-ablation clinical events, translating to a 15 percent reduction in post-ablation management costs ($3,402 savings per patient) in the year after ablation versus patients treated with a non-contact force ablation catheter.
The magnetic sensor of the catheter integrates with the Abbott EnSite Precision cardiac mapping system, providing the ability to collect both magnetic and impedance (resistance) data so physicians can create more life-like models of the heart. It also uses the Abbott FlexAbility catheter platform's advanced ergonomic handle-shaft combination, which is designed for reach, maneuverability and 1:1 torque.
When utilized with EnSite Precision, the TactiCath Contact Force Ablation Catheter, Sensor Enabled can improve workflow efficiency in the lab. The system enables automated guidance of lesion marking, verification of the ablation catheter stability, the ability to automatically record the precise location of the catheter tip during radiofrequency energy application, the capability to add mapping points based on Contact Force, and the ability to review and identify any potential gaps by viewing specific lesions from the display list.
Currently the TactiCath Contact Force Ablation Catheter, Sensor Enabled is available in select markets in Europe, with full market release expected in the third quarter. Abbott is pursuing approval in the U.S.
For more information: www.abbott.com


 January 22, 2026
January 22, 2026 









