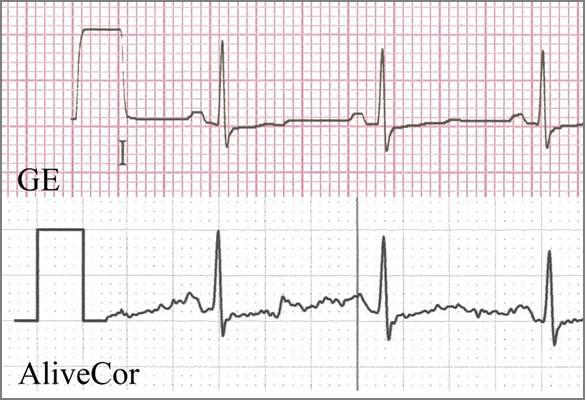
In 62 patients, two physicians found the QRS morphology to be the same between the two devices a standard 12-lead ECG and though the AliverCor iPhone-based ECG.
May 18, 2012 — AliveCor, the developer of a breakthrough mobile electrocardiogram (ECG) recorder, announced results from a study that demonstrated the accuracy of the company’s iPhone-based device, by comparing its Lead I to Lead I from a conventional 12-lead ECG. The study found that the iPhone-based event recorder is an accurate clinical tool for ECG assessment and could prove to be a new tool for allowing immediate recording and analysis of an ECG rhythm.
The AliveCor smartphone ECG is an innovative, investigational medical wireless device which incorporates electrodes into a wireless case that snaps onto the back of a smartphone. The device allows for wireless single-lead recording of rhythm strips (from 30 seconds to continuous reading) that are stored securely in the cloud and then within the app itself. The ECGs are wirelessly uploaded for immediate interpretation anywhere using simple Web browsers. The AliveCor smartphone ECG is designed to work in conjunction with a range of mobile platforms, including iPhone, iPad and Android devices.
The results from this study were presented today in a poster presentation in the Innovation Abstract category at the "Heart Rhythm Society’s 33rd Annual Scientific Sessions" in Boston (Abstract 12-IA-9662-HRS) by Paul Garabelli, M.D., of the University of Oklahoma Health Sciences Center. The authors of the abstract also include: David Albert, M.D., founder and chief medical officer of AliveCor and Dwight Reynolds, M.D., chief of cardiology at the University of Oklahoma Health Sciences Center.
“We are encouraged by the continued validation of AliveCor’s innovative technology, which upon anticipated marketing approval will improve early diagnosis of arrhythmias and overall public awareness of health metrics,” commented Albert, inventor of the technology. “We envision that our technology will significantly benefit patients who need consistent and more convenient monitoring.”
Twelve-lead ECGs (Mac5500, GE Healthcare) with gel-based electrodes and Lead I ECGs (AliveCor ECG) were recorded in 67 patients. Five patients with pacemakers had the iPhone placed directly on the chest to record a V3-V4 rhythm strip, and two physician reviewers cross-validated these strips for pacing spikes. All recordings were performed with the iPhone in Airplane mode so no radio frequency interference was generated.
In 62 patients, two physicians found the QRS morphology to be the same between the two devices, though the iPhone-based ECG had more baseline noise than the standard ECG. The mean/SD of the R-wave amplitudes for the standard and iPhone recordings respectively were 0.77/0.24 (mV) and 0.78/0.24 (mV), P<0.0001. In five patients with pacemakers, pacing spike artifacts were clearly identified in all five enabling verification of pacing capture.
For more information: www.alivecor.com



 July 22, 2025
July 22, 2025 



