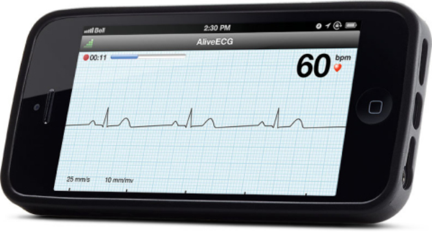
June 24, 2013 — AliveCor Inc.’s Heart Monitor for iPhone 4, 4S and 5 is now available for purchase in the United Kingdom and Ireland. It is the first CE-marked mobile device–based electrocardiogram (ECG) monitor that is used to record, display, store and transfer single-channel ECG rhythms. It is available to medical professionals, patients and health-conscious individuals at Amazon.co.uk.
“The simplicity and convenience of the AliveCor device is extraordinary,” said John Camm, M.D., professor of clinical cardiology, SGUL. “The Heart Monitor increases the accessibility of ECGs, giving everyone the ability to record anytime, anywhere.” Camm continued, “You can now capture and review clinical-grade ECG rhythm strips within seconds, without special preparation and without attaching leads.”
AliveCor’s Heart Monitor snaps onto the back of an iPhone to record, display, store and transfer single-channel ECG rhythms wirelessly with the corresponding, free AliveECG app. Recorded rhythm strips can be of any duration, and are stored in the app and securely in the cloud in PDF format for reviewing, analysis and printing through AliveCor’s website. Cloud storage is compliant with the UK Data Privacy Act and the Data Protection Directive in the European Union. ECG data is accessible from any Web browser.
For more information: www.alivecor.com


 January 15, 2026
January 15, 2026 









