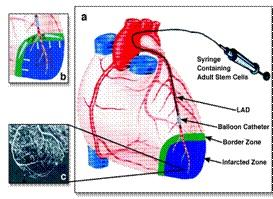
January 26, 2012 — Amorcyte LLC, a NeoStem company, announced the enrollment of the first patient in the Amorcyte PreSERVE Phase II trial for acute myocardial infarction. The study is a multicenter, randomized, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of infarct-related artery infusion of AMR-001, an autologous bone marrow derived cell therapy enriched for CD34+ cells.
AMR-001 is administered five to 11 days post-stent placement in patients diagnosed with an ST segment elevation myocardial infarction (STEMI) with ejection fraction less than or equal to 48 percent, as determined by cardiac magnetic resonance imaging measured after recovery from myocardial stunning. Approximately 160 subjects, age 18 and older, will be randomized 1:1 between the treatment group and control group. Progenitor Cell Therapy LLC, also a NeoStem company, will support the manufacturing, product supply and logistics for the trial.
AMR-001 represents the first compound in this class of cell therapies to have a highly defined cell population and an identified biologically effective therapeutic dose, both of which tie back to the biological mechanism of action that the outcomes of the current study are intended to demonstrate. The Amorcyte therapy is being developed initially for the preservation of heart muscle function for approximately 160,000 American patients who sustain a heart muscle damaging STEMI annually.
For more information: www.amorcyte.com, www.neostem.com


 January 05, 2026
January 05, 2026 









