October 6, 2010 – A new statement from the American Heart Association says arteriotomy closure devices are reasonable to use, but their benefits should be weighed against the risk of complications.
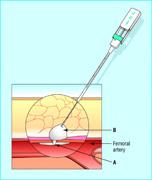
Arteriotomy, the process of creating a hole in an access artery through which instruments are inserted, is the first step in procedures such as angiography or percutaneous coronary intervention (PCI). Closing up the artery is a concern due to possible bleeding complications.
Arteriotomy closure is typically done with manual compression, but more devices are entering the market to offer potential improvements over manual closure. The statement is published in Circulation: Journal of the American Heart Association.
The statement authors conclude that the devices are reasonable to use - and they offer recommendations for their use based on available evidence - but their benefits should be weighed against the risk of complications in patients. Patient-specific factors such as age, gender and disease severity should be considered before using an arteriotomy closure device.
The statement also includes recommendations for future trials and end points needed to inform clinical practice.
In the United States, 1.3 million PCI procedures were performed in 2006, the most recent year for which data is available.
For more information: www.heart.org


 October 27, 2021
October 27, 2021 







