December 8, 2015 — Biotronik announced the first patient enrollments to the B3 clinical trial. The study will evaluate the potential clinical benefit of the physiologic rate response sensor Closed Loop Stimulation (CLS) in atrial fibrillation (AF) patients. It will assess whether pacemakers and implantable cardioverter-defibrillators (ICDs) with CLS can reduce the rate of stroke and improve patient outcomes.
AF is one of the most common cardiac arrhythmias; a significant risk factor for stroke, it is estimated to cause approximately 15 percent of all ischemic strokes and as many as 30 percent of strokes for patients in their eighties.
“AF is a huge clinical challenge, affecting over 20 million patients in Europe and the U.S. alone,” commented first implanter and international coordinator for the study Valeria Calvi, M.D., University of Catania, Ferrarotto Hospital, Italy. “I am very excited to begin this groundbreaking trial. We hope to demonstrate that the physiological heart rate modulation induced by CLS in sick sinus disease patients can control clinically relevant AF episodes. CLS may well improve clinical outcomes and lower the incidence of stroke in this particularly challenging patient population.”
The large multicenter trial will include 52 study centers in Italy, Spain, China, Taiwan, Korea, Singapore, Malaysia and India. Investigators will randomize 1,308 patients with a pacemaker or ICD indication who require dual-chamber pacing due to sinus node disorder (SND) into two groups. Patients in each study arm are to receive a Biotronik pacemaker or ICD with CLS turned either on or off. The trial’s primary endpoint is the time to first onset of new clinically relevant AF episodes or thromboembolic events, and there will be a follow-up period of three years. As a secondary endpoint, the study will investigate whether a subgroup of patients remotely monitored using Biotronik Home Monitoring is associated with an even further reduced risk of stroke and persistent AF.
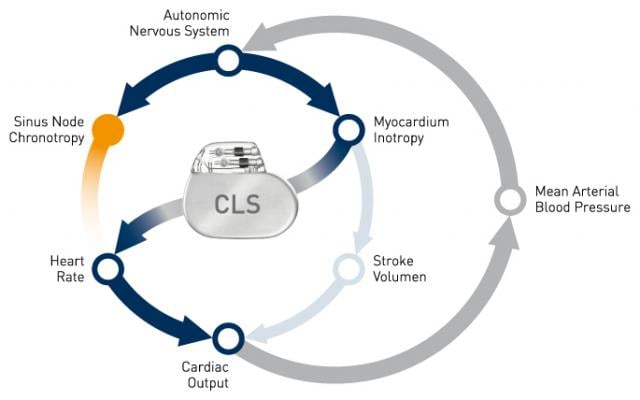
CLS rate adaptive technology has been available in select pacemakers for two decades, and more recently in ICDs and CRT devices. CLS integrates into the natural cardiovascular control system and determines the appropriate heart rate based on intracardiac impedance measurements. These measurements reflect changes of the cardiac contraction dynamics in reaction to information coming from the autonomic nervous system. In doing so, CLS provides optimal heart rates under varied circumstances, even during acute mental stress.
For more information: www.biotronik.com


 December 19, 2025
December 19, 2025 









