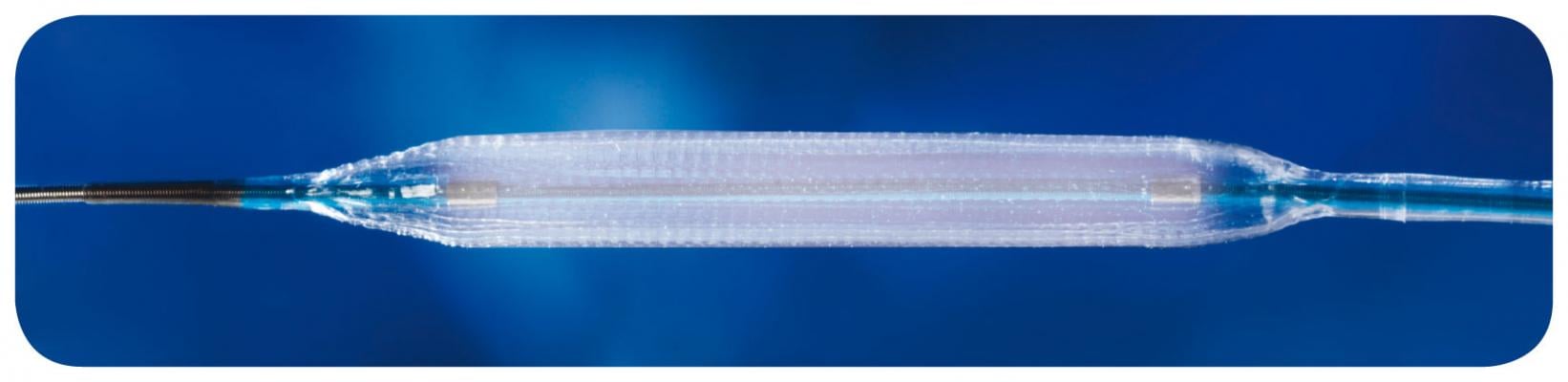
March 20, 2012 - MarBlue Medical announced the global product launch of its CE-marked drug-eluting balloon (DEB) Protege as well as its Pioneer cobalt chrome (CoCr) stent on a DEB. The full market launch of the new DEB technology is a major step forward in Blue Medical's quest to improve the quality of life of patients with cardiovascular diseases, while at the same time reducing procedure costs.
Blue Medical's DEB therapy delivers a controlled dose of paclitaxel to the coronary artery during balloon angioplasty. The combined device with a coronary stent, the DEB offers a one-time shot of the drug, instead of continuous drug delivery from a drug-eluting stent. This significantly reduces the period in which antiplatelet medication could be required. Low major adverse cardiovascular events (MACE) rates and low late lumen loss indicating the efficacy and safety of the Protege and Pioneer DEB products are confirmed by the clinical data of Blue Medical's PIONEER human trial.
"Increased late stent thrombosis risk and the long-term dual antiplatelet medication which are associated with the majority of current drug-eluting stents is a concern in treating our patients today," said primary investigator of the PIONEER study Peter Smits, M.D., head of interventional cardiology at the Maasstad Hospital, Rotterdam, The Netherlands. "DEB therapy in combination with bare metal coronary stents offers a potential solution for reducing late lumen loss without the need for long-term dual-antiplatelet medication, improving patient comfort and reducing risks for bleeding and stent thrombosis."
"Our extensive research effort combined with human evaluation ensures a highly reliable science-based and effective DEB technology," said Ronald Horvers, CEO of Blue Medical. "The critical and extensive review of the Dutch Medicine Evaluation Board and notified body BSI enables us to provide an attractive alternative for treating coronary disease."
For more information: www.bluemedical.com


 November 24, 2025
November 24, 2025 









