October 2, 2020 — Cardiac Dimensions announced the company has closed a $17.5 million Series C financing that will be used to help expand the sales of the Carillon Mitral Contour System, a transcatheter device used to treat functional mitral regurgitation (FMR) in patients with heart failure.
All existing shareholders participated in the round including Aperture Venture Partners, Arboretum Ventures, Hostplus, Life Sciences Partners, Lumira Ventures, and M. H. Carnegie & Co.
The financing will be used to accelerate commercial sales of Cardiac Dimensions' Carillon Mitral Contour System in Europe and allow for expansion into other geographies such as Australia. Earlier this month, Australia's Therapeutic Goods Administration approved the Carillon System for commercial use in FMR patients with mild to severe mitral regurgitation.
"The Carillon System is a transformative product which has shown great success in helping an extremely large patient population with very few options," said Rick Wypych, CEO and president of Cardiac Dimensions. "The new round of financing will allow for continued growth of sales in Europe and provide capital for our expansion efforts into other geographies–such as Australia. We thank our investors for their continued support as our business shows excellent results despite the challenging environment."
An estimated 26 million people, worldwide, suffer from heart failure and of those, approximately 70 percent have functional mitral regurgitation.[1] FMR is due to the mitral valve not closing tightly, resulting in blood flowing or leaking backwards through the mitral valve. This further decreases the amount of oxygenated blood that is pumped out to the body, increases the symptoms associated with heart failure, and decreases the quality of life of those who have been diagnosed.
"We're excited to support the expansion of sales of the Carillon System," said Trevor Moody of M. H. Carnegie & Co. "Cardiac Dimensions' innovative efforts and dedication to expanding access to the Carillon System will soon allow people worldwide to benefit from this therapeutic innovation."
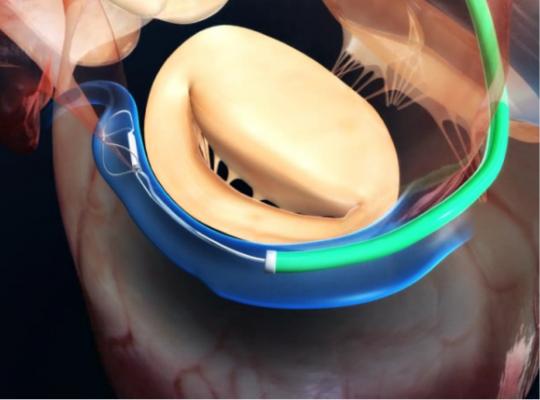
The Carillon System offers a simple right heart approach to transcatheter mitral valve repair designed to reshape the anatomy and function of the mitral apparatus from the coronary sinus. Distal and proximal anchors, connected by a shaping ribbon, utilize the heart's venous anatomy to cinch the mitral apparatus without compromising the valve or future treatment options.[2,3] The Carillon System is designed to treat the primary cause of functional mitral regurgitation in patients with MR grades 2+, 3+ and 4+ and is the first and only device to demonstrate a reduction in regurgitant volume and favorable left ventricular remodeling in a randomized sham-controlled clinical trial of percutaneous valve therapy.[4,5,6] The Carillon System is CE-marked and has been implanted in over 1,200 patients in the U.S., Europe, Australia, Turkey and the Middle East. The Carillon System is currently being studied in The CARILLON Trial pivotal trial and limited to investigational use in the United States.
For more information: cardiacdimensions.com
References:
3. Latib, A. "Coronary Sinus Annuloplasty." New York, Montefiore Medical Center.
6. Sievert, H. 2018. REDUCE-FMR: A Sham-controlled Randomized Trial of Transcatheter Indirect Mitral Annuloplasty in Heart Failure Patients with Functional Mitral Regurgitation. Presented at TCT 2018, San Diego, CA.


 November 14, 2025
November 14, 2025 









