March 25, 2008 - Medical Simulation Corp. will present a comprehensive onset-of-symptoms-to-cath lab scenario at the SCAI/ACC national conference in Chicago, IL, March 30-April 1, in a presentation that follows a patient suffering from myocardial infarction from door-to-cath lab.
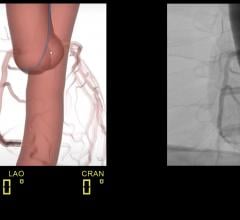
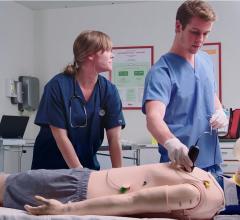
The presentation, which takes place on Monday, March 31 at 1:30 p.m. in the Simulation Pavilion 360 Degree Theater, is said to represent a milestone in simulation education, as multiple simulators are incorporated to deliver a comprehensive training experience. The simulated scenario will follow a patient suffering from an acute myocardial infarction (AMI) from the onset of symptoms and through the EMS and ER process of care using a human patient simulator. The patient will then be transferred to the cath lab for a cardiovascular intervention using MSC’s endovascular simulator.
“By incorporating the complete process of care, this scenario will highlight the complexities surrounding the treatment of a cardiac patient from the onset of symptoms to the cath lab,” said Mark A. Turco, M.D., FACC, FSCAI, director of the Center for Cardiac & Vascular Research at Washington Adventist Hospital in Takoma Park, MD. “A primary initiative in improving care of AMI patients is finding ways to speed the door-to-cath lab time with the overall goal of saving more heart muscle. Simulation training provides the ideal platform to address these challenges in a realistic environment.”
“MSC is proud to continue to deliver more advanced simulation training opportunities to healthcare providers,” said Bill Younkes, MSC president. "As we move closer to simulating the entire process of care, education empowered by simulation can have a greater impact on improving patient outcomes and safety."
For more information: www.medsimulation.com


 March 11, 2022
March 11, 2022