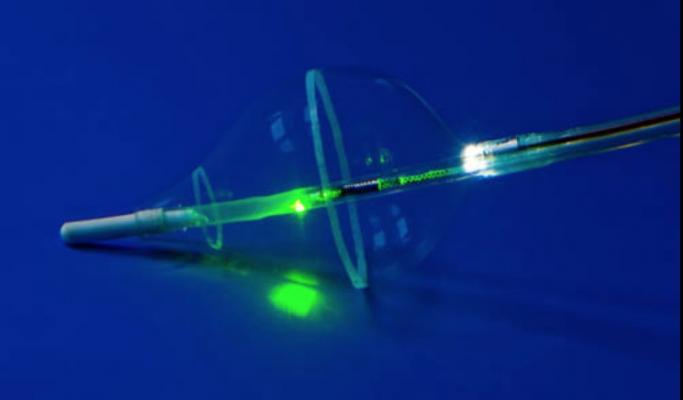
January 25, 2017 — CardioFocus Inc. recently announced the initial clinical evaluation of the HeartLight Excalibur Balloon, a next-generation technology designed for the treatment of atrial fibrillation (AF). The Excalibur Balloon leverages the universal balloon design of the HeartLight Endoscopic Ablation System and introduces an advanced feature set that optimizes the speed and magnitude of target tissue contact during pulmonary vein isolation (PVI) procedures.
A live patient case featuring the HeartLight Excalibur Balloon was included in the session, "Advances in Catheter Ablation for AF and Left Atrial Appendage Closure," at the 22nd Annual AF Symposium on Friday, Jan. 13. The live case was performed by Prof. Petr Neužil, Lucie Šedivá, M.D.; Jan Petrů, M.D.; and Jan Skoda, M.D., from Na Homolce Hospital, in Prague, Czech Republic.
"The HeartLight Excalibur Balloon conforms effectively to the range of anatomies that we encounter during PVI procedures," said Petrů, head of the electrophysiology laboratory. "It is also very responsive, which may result in both better procedural efficiencies and potentially better patient outcomes. We are very pleased with the experience thus far."
In addition to a more compliant construction that enables adaptive vein conformance, the Excalibur Balloon also incorporates proprietary Dynamic Response technology. This feature makes the balloon highly responsive to a range of user techniques and amounts of pressure applied, while optimizing vein contact. The result is a design meant to maximize the engagement of the balloon with the pulmonary veins, while decreasing the time required to complete ablation procedures.
The balloon is undergoing an initial clinical evaluation in Europe as part of a broader development program that seeks to confirm its design objectives.
For more information: www.cardiofocus.com


 February 06, 2026
February 06, 2026 









