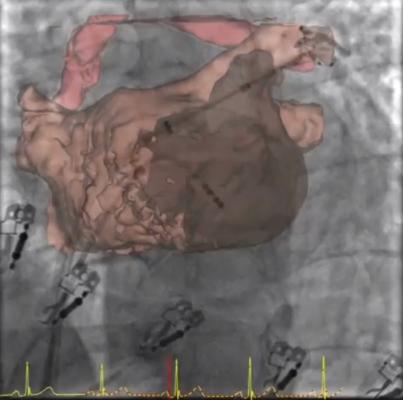
February 27, 2014 — Apama Medical is a privately held medical device company formed by Shifamed's medical incubator. It received Chinese notice of allowance for claims covering their low profile electrode assembly that enables the attachment of flexible electrodes to an ablation balloon for energy delivery and monitoring. The Chinese patent will complement an existing patent (U.S. Patent No. 8,295,902) giving Apama broad claim coverage for electrode-based ablation balloons in the United States and China for the treatment of atrial fibrillation (AF). Utilizing this patented electrode technology enables Apama to design an ablation catheter that performs a full pulmonary vein (PV) ablation and rotor ablation in a single-shot.
The global electrophysiology (EP) mapping and ablation market is estimated to reach $4.3 billion in 2017 and is the fastest growing cardiac rhythm management market segment. Worldwide catheter based AF ablation is estimated to grow from 180,000 procedures in 2009 up to 827,000 procedures by 2017. Fundamental barriers to adoption of AF ablation procedures have been attributed to technical complexity and length of procedure time. As a result, there is a large underserved market.
For more information: www.shifamed.com


 February 06, 2026
February 06, 2026 









