February 14, 2019 – Circle Cardiovascular Imaging and Galgo Medical recently announced an agreement for distribution and joint development with respect to magnetic resonance imaging (MRI) scanner application products and workflows, with corresponding post-processing capabilities. The focus will be to improve cardiac MRI workflows and diagnostic tools to expand accessibility and use of the modality to electrophysiology procedures such as atrial fibrillation and ventricular tachycardia ablations.
The announcement was made at the Society for Cardiovascular Magnetic Resonance (SCMR) 2019 meeting, Feb. 6-9 in Bellevue, Wash.
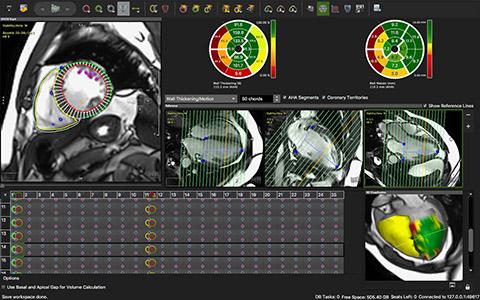
Circle’s imaging platform, cvi42 is a cardiovascular imaging reading and reporting solution for cardiac MR, cardiac computed tomography (CT) and cardiac interventional planning. cvi42 provides physicians the tools to accurately quantify and diagnose complex cardiovascular diseases while improving patient outcomes and the effective utilization of healthcare resources. Currently at version 5.10, cvi42 utilizes machine learning and big data, with the machine results demonstrating capabilities within the same range as expert human readers.
Galgo Medical SL is a Barcelona-based company that emerged from the Department of Information Technologies (DTIC) at the Universitat Pompeu Fabra (Barcelona, Spain). Its ADAS 3D family of products is intended for the identification of arrhythmia substrate and the planning of ablation procedures using cardiac MRI and CT.
For more information: www.circlecvi.com, www.adas3d.com


 July 31, 2024
July 31, 2024 









