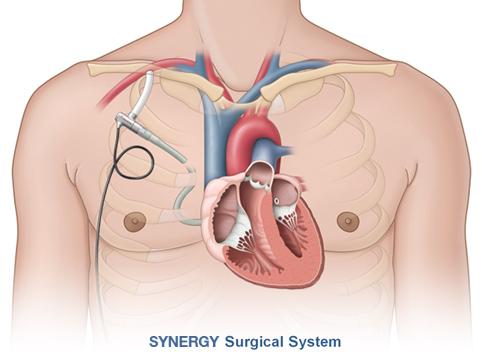
May 20, 2013 — CircuLite Inc. announced that it has received approval from the Federal Agency for Medicines and Health Products in Belgium to commence the CE mark trial of the Synergy IC Circulatory Support System, the first mechanical support system that does not require major surgery. The Synergy IC System is based on the surgical Synergy Circulatory Support System — the world’s smallest commercially available circulatory support pump — which is designed to treat ambulatory chronic heart failure patients (INTERMACS ?4).
While the Synergy IC Circulatory Support System uses the same superficially placed micro-pump platform as the surgical system, it is the first implantable circulatory support system whose Inflow Cannula is designed to be implanted by a cardiologist using standard interventional techniques. The procedure is designed to further reduce the invasiveness of implantation, and thereby reduce the rate and severity of adverse events.
“Imagine being able to implant a permanent mechanical support device in a patient with chronic heart failure without major surgery,” said Daniel Burkhoff, M.D., Ph.D., chief medical officer of CircuLite and adjunct associate professor of medicine at Columbia University Medical School. “If successful, this approach will usher in a new era in mechanical circulatory support, much the same way TAVR did for aortic valve replacement.”
The multi-center CE mark trial will enroll up to 20 patients, starting in Belgium and expanding to two additional European clinical sites. Clinical status, end organ function, exercise tolerance, functional capacity and quality of life will be assessed post-implantation. Patient screening has commenced at the University Hospitals Leuven, Belgium.
“With an even less invasive, interventional procedure performed in the cath lab or hybrid OR, we will be making the Synergy System more accessible, more convenient and potentially safer for patients,” said Paul Southworth, chief executive officer of CircuLite.
Burkhoff will be presenting an abstract on The Synergy IC System at EuroPCR in the Cardiovascular Innovation Pipeline — Novel Interventional Approaches for Heart Failure session, to be held on Thursday, May 23, 2013 in Room 352A of the Palais des Congrés in Paris.


 June 19, 2024
June 19, 2024 









