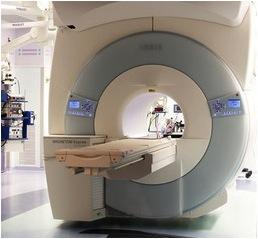
August 19, 2013 — IMRIS Inc. announced that a craniotomy recently performed on a 5-year-old boy with epilepsy was the 500th neurosurgical case at Cook Children's Medical Center in Fort Worth, Texas, using its intraoperative magnetic resonance imaging (iMRI) system.
Cook Children's was the second children's hospital in the United States and fourth hospital worldwide with an IMRIS system that features a high-field MRI, which moves between surgical and diagnostic rooms using ceiling-mounted rails.
"Using the intraoperative MRI allows us to clearly differentiate the tissue targeted for removal whether resecting for tumor or epilepsy," said John Honeycutt, M.D., medical director of the Cook Children's Department of Neurosurgery, who conducted the milestone operation. "With this enhanced vision, we can remove it more completely while avoiding critical structures and minimize returns to the operating room for repeat procedures."
The IMRIS Visius Surgical Theatre with iMRI allows neurosurgeons to take high-quality MR images during surgery to see the area of the brain they are operating on in exquisite detail, see tumor size and shape and distinguish between healthy and unhealthy tissue more easily. The surgeon can take images before completing the surgery and check if additional tumor or abnormal tissue removal is needed to reduce risk of re-operation. Unlike other iMRI systems, the scanner moves to the patient inside the operating room (OR) so the surgical team can maintain optimal surgical positioning, access and techniques.
The Cook Children's Visius iMRI was the first IMRIS suite installed with two adjoining rooms - one intended for surgery and the other for diagnostic scanning. In addition to tumor resection and epilepsy treatment, the surgical theater has been used for cases of chiari malformation, deep brain stimulation, hydrocephalus and other neurological conditions.
For more information: www.cookchildrens.org


 January 21, 2026
January 21, 2026 








