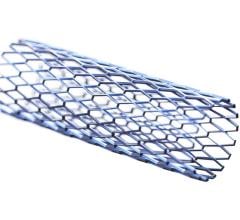
March 21, 2008 - Cordis Corp. has received 510(k) marketing clearance from the FDA for the S.M.A.R.T. Nitinol Stent Transhepatic Biliary System for lengths of 120 mm and 150 mm, the company reported at the 33rd Annual Scientific Meeting of the Society of Interventional Radiology (SIR) meeting.
These stents are indicated for use in the palliative treatment of malignant strictures in the biliary tree that can restrict the flow of digestive fluids and compromise digestion.
Available for the first time in the U.S. in the new lengths, the stents have demonstrated accurate stent placement, which may decrease the need for additional stents to cover the full narrowing of the bile duct. The safety and effectiveness of this device for use in the vascular system have not been established.
For more information: www.cordis.com

 November 13, 2025
November 13, 2025