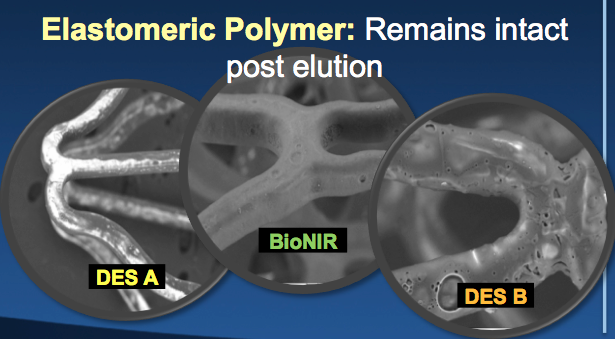
Imaging of stent struts after expansion, showing the elastic polymer used on Medinol’s BioNIR stent does not crack or flake off, possibly leading to better patient outcomes.
November 7, 2016 – The large multinational randomized BIONICS study found that a novel elastic polymer coated ridaforolimus-eluting stent (Medinol’s BioNIR stent) was non-inferior to a zotarolimus-eluting stent (Medtronic’s Resolute) for one-year clinical outcomes in a broad, less selected ‘more comers’ population. Results of this trial will be submitted to the FDA for U.S. approval of this novel drug-eluting stent.
Findings were reported at the 28th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.
“One-year clinical outcomes from the BIONICS study clearly show that the ridaforolimus-eluting stent was non-inferior to the zotarolimus-eluting stent with identical target lesion failure rates,” said principal investigator, David E. Kandzari, M.D., director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta, Ga. “In addition, it had a low stent thrombosis rate with no events beyond 30 days.”
The ridaforolimus-eluting stent is a thin-strut (90 microns) cobalt chromium stent that elutes the rapamycin analogue ridaforolimus from a durable elastomeric polymeric coating. The multicenter randomized trial was conducted at 76 medical centers in the U.S., Canada, Europe, and Israel and enrolled a broad range of patients with coronary artery disease including patients with non-ST elevation MI (NSTEMI) as well as complex lesions. The primary endpoint was target lesion failure (TLF) at one year, the composite rate of cardiac death, target vessel myocardial infarction (TV-MI), and ischemia driven target lesion revascularization (ID-TLR). Secondary endpoints were one year major adverse cardiovascular events (MACE), target vessel failure (TVF) and individual component endpoints, definite/probable stent thrombosis and procedural success.
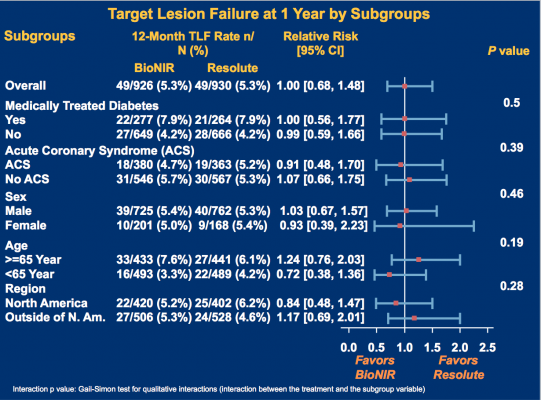
A total of 1,919 patients were randomized 1:1 to the BioNIR ridaforolimus-eluting stent (n= 958, 1,275 lesions) or Resolute zotarolimus-eluting stent (n=961, 1,277 lesions) stent. The baseline clinical characteristics were well balanced between the two arms, as were angiographic baseline characteristics with the exception of severe calcification (13.3% in the BioNIR group vs. 10.5% in the Resolute group, P=0.03).
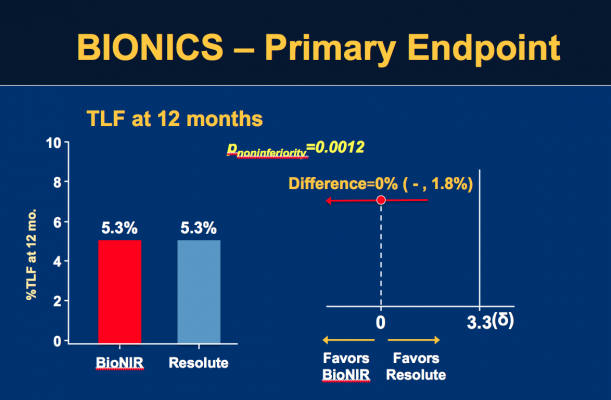
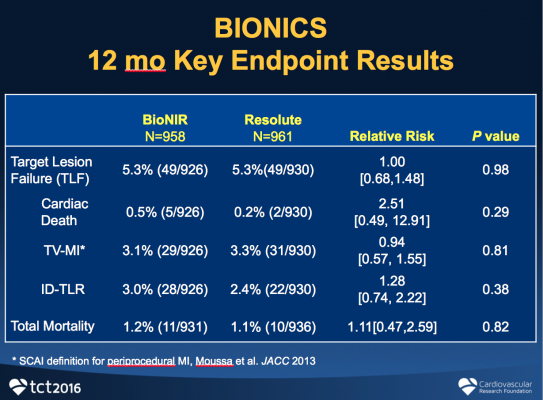
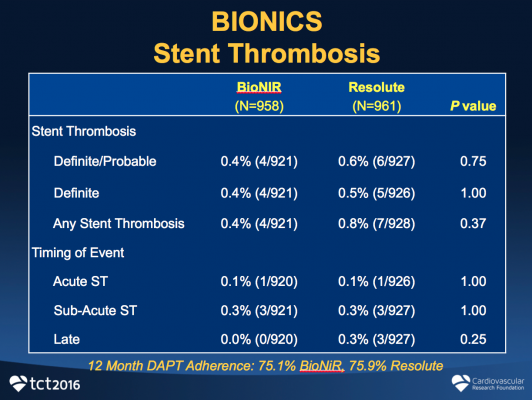
The primary endpoint of TLF at one year was identical for both at 5.3% (P=0.98). In addition, the rates of cardiac death (0.5 vs. 0.2%, P=0.29), TV-MI (3.1 vs. 3.3%, P=0.81) and ID-TLR (3 vs. 2.4%, P=0.38) were similar. Definite/probable stent thrombosis was 0.4% (4/921) for the ridaforolimus-eluting stent and 0.6% (6/927) for the zotarolimus-eluting stent (P=0.75). Device success was 98.3% for the ridaforolimus-eluting compared to 99.5% for the zotarolimus-eluting stent (P=0.004).
The BIONICS trial was funded by Medinol and designed and conducted by the CRF Clinical Trials Center in collaboration with Novella Clinical which provided site management. Kandzari reported grant or research support from Abbott Vascular, Boston Scientific, Medtronic, Biotronik, St. Jude Medical/Thoratec, and Ablative Solutions, and consulting fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and St. Jude Medical.
For more information: www.crf.org


 November 14, 2025
November 14, 2025 









