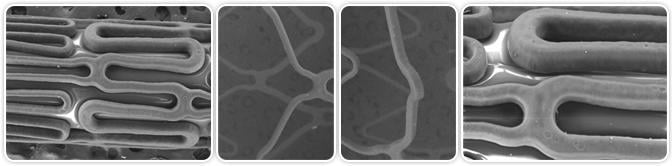
The eDES elastomer coating before and after expansion. Image courtesy of Medinol Inc.
October 26, 2015 — Medinol announced in September the completion of enrollment in its BIONICS trial to evaluate the safety and effectiveness of a new coronary stent system, the first-ever elastomeric drug eluting stent (eDES).
The BIONICS trial is a global, prospective, randomized, multicenter, clinical trial enrolling 1,918 patients in the United States, European Union, Canada and Israel. The results will be submitted to the U.S. Food and Drug Administration (FDA) for the U.S. approval of the eDES for the treatment of patients with narrowing or blockage of their coronary arteries.
The device is coated with an elastomer that maintains a smooth and uniform stent surface designed to prevent the cracking or peeling that may occur with brittle polymers used in other DES during the stent crimping and deployment processes. The stent is comprised of cobalt chromium and manufactured and coated in flat panels utilizing Medinol's patented QualitySurface technology and contains a "limus" family drug, intended to prevent restenosis. The eDES design is based on the clinically proven NIRxcell stent architecture intended to enhance conformability, scaffolding and radial strength. The delivery system features a distinctive spring tip that is simultaneously more pushable and flexible than the plastic tips used on other delivery systems.
"The design of eDES enhances stent deliverability and conformability for the treatment of complex coronary disease. The system is unique from other products currently being offered, and will be a great addition to our current treatment options." said David Kandzari, M.D., FACC, director of interventional cardiology at Piedmont Heart Institute, Atlanta, and principal investigator for the BIONICS trial.
Additionally, Kandzari was impressed with several aspects of the study. "One of the distinctive aspects of the study is how inclusive the enrollment criteria is, allowing for almost all patient populations to be included in the study, and making it very relevant to the populations we see in our practices today. We look forward to getting the results after follow-up," he said.
The BIONICS clinical trial is a 1:1 randomized study comparing the eDES System to Medtronic's Resolute Integrity Stent System. The primary endpoint of the study is target lesion failure (TLF) as determined at 12 months. The eDES System is an investigational device and is not available for commercial sale.
For more information: www.medinol.com


 November 14, 2025
November 14, 2025 









