May 2, 2019 — Abiomed announced that, on April 26, the U.S. Food and Drug Administration (FDA) approved initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial. The prospective, multi-center, two-arm trial plans to enroll 668 patients undergoing treatment for a STEMI heart attack. Half the patients will be randomized to receive delayed reperfusion after 30 minutes of left ventricular unloading with the Impella CP. The other half will receive immediate reperfusion, the current standard of care.
The trial will test the hypothesis that unloading the left ventricle for 30 minutes prior to reperfusion will reduce myocardial damage from a heart attack and lead to a reduction in future heart failure-related events. Myocardial damage can lead to an infarct, and every 5 percent increase in infarct size is associated with a 20 percent increase in relative hazard for all-cause mortality or hospitalization for heart failure within one year after a primary percutaneous coronary intervention (PCI)[1]. Coronary artery disease is the No. 1 cause of death in the United States — 47 percent of women and 36 percent of men over the age of 45 will die within five years of their first heart attack[2].
“The STEMI DTU Pivotal Trial has the potential to improve the standard of care, slow the growing epidemic of heart failure and improve outcomes for millions of heart attack patients. This trial is the first of its kind to focus on ventricular unloading as part of a therapeutic approach for heart attacks without cardiogenic shock and could lead to a paradigm shift in the way heart attack patients are treated worldwide,” said Navin Kapur, M.D., the study’s co-principal investigator and the executive director of the CardioVascular Center for Research and Innovation (CVCRI) at Tufts Medical Center, Boston.
Trial protocol requires each site to “roll-in” two patients (one in each arm) to test the study protocol before beginning enrollment. The trial allows for an adaptive design, which permits adjustments to the study sample size after an interim analysis. Enrollment begins in October 2019 and is expected to end in 3-4 years, 2022-2023.
The primary endpoint for the STEMI DTU trial will be infarct size as a percent of left ventricular mass, measured at 3-5 days using cardiac magnetic resonance imaging (MRI). The key secondary effectiveness endpoint will track a composite of the primary endpoint and the following:
-
Cardiogenic shock after 24 hours post-enrollment out to 30 days;
-
Cardiovascular mortality at 24 months;
-
Heart failure requiring hospitalization at 24 months;
-
Implantable cardioverter-defibrillator (ICD) and cardiac resynchronization therapy (CRT) placement at 24 months; and
-
The trial is powered for multiple other secondary endpoints and has numerous exploratory endpoints, such as infarct size to area at risk
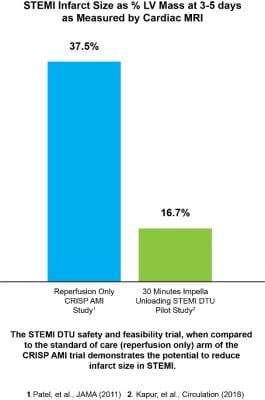
The pivotal trial will build on the promising results of the successful STEMI DTU safety and feasibility trial, which met its goal by demonstrating it is safe and feasible to conduct a study of 30 minutes of unloading with delayed reperfusion. The accompanying chart (see below) compares the safety and feasibility trial results to the CRISP AMI trial’s standard of care arm (reperfusion only), and demonstrates how 30 minutes of unloading prior to reperfusion has the potential to reduce infarct size in STEMI.
The pivotal trial will be overseen by a steering committee of five expert cardiologists and clinical trialists. They are Kapur and:
-
William O’Neill, M.D., medical director of the Center for Structural Heart Disease at Henry Ford Hospital;
-
Jeffrey Moses, M.D., professor of medicine at Columbia University Medical Center and director of interventional cardiovascular therapeutics at Columbia University Medical Center;
-
Gregg Stone, M.D., professor of medicine at Columbia University College of Physicians and Surgeons and director of cardiovascular research and education at the Center for Interventional Vascular Therapy at New York-Presbyterian Hospital/Columbia University Medical Center; and
-
James Udelson, M.D., chief of the division of cardiology at Tufts Medical Center.
The trial is sponsored by Abiomed. Impella heart pumps are not FDA-approved for use in STEMI patients without cardiogenic shock.
For more information: www.abiomed.com
References
1. Stone, et al., Relationship Between Infarct Size and Outcomes Following Primary PCI, JACC, 2016


 January 05, 2026
January 05, 2026 









