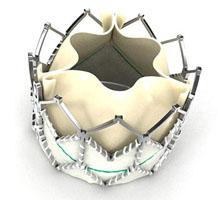
September 23, 2010 – The U.S. Food and Drug Administration (FDA) has conditionally approved the first part of a trial testing a new transcatheter heart valve.
The first cohort of the Partner II trial will study the Edwards Sapien XT transcatheter heart valve and the low-profile NovaFlex transfemoral delivery system. Partner II will study up to 450 patients with severe, symptomatic aortic stenosis using two-to-one randomization, where for every two patients who receive the valve delivered transfemorally, one will receive standard therapy.
A second patient cohort will compare traditional open-heart surgery with the Sapien XT delivered either transfemorally or transapically.
The transcatheter valve received a CE mark in March 2010, but is not yet commercially available in the United States.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









