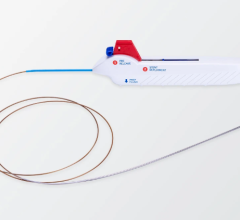
The FDA has given CoAxia Inc. approval to move forward on a safety and feasibility study for the NeuroFlo perfusion augmentation therapy in ischemic stroke patients who present for treatment as late as 24 hours after stroke onset. Perhaps the first interventional device trial approved for this patient population, the study will enroll up to 25 patients and the results will determine future expansion to a pivotal study in late presenting stroke patients.
"This study represents another important step in applying medical devices to the treatment of acute stroke,” said Souvik Sen, M.D., director of the Stroke Service at the University of North Carolina and a co-author of the study. “There are no treatments currently approved to treat stroke patients in this eight- to 24-hour time window after symptom onset. If NeuroFlo can safely treat the large number of patients who present in this time window, it will have a major impact in stroke treatment."
The SafeFlo24 trial will evaluate the safety of the NeuroFlo perfusion augmentation catheter in stroke patients presenting for treatment between eight and 24 hours after last known to be normal. The study’s other co-authors are Lee Schwamm, M.D. from Massachusetts General Hospital, Maxim Hammer, M.D. from University of Pittsburgh Medical Center and David Liebeskind, M.D. from UCLA Medical Center. In addition to these four centers, the University of Rochester, New York, the University of Alberta, Edmonton and the University of Calgary, Calgary, will participate in the study. CoAxia expects patient enrollment to begin in the first quarter of 2007.
For more information visit www.coaxia.com


 February 02, 2026
February 02, 2026