Image courtesy of FEops
January 26, 2015 — FEops announced the closing of a €1.3 million (~$1.9 million USD) series A financing round, led by Capricorn Venture Partners and PMV. The funding will be used to support the launch of FEops’ first product, TAVIguide, in key markets in Europe and the United States.
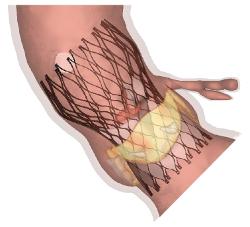
TAVIguide is a cloud based pre-operative planning service for transcatheter aortic valve implantation (TAVI). Part of the proceeds will be dedicated to advance clinical development of the technology and diversify the pre-operative planning services offered to include additional cardiovascular interventions.
Current TAVI planning relies on pre-operative imaging such as echography or computed tomography (CT), but accurate measurements of the pre-operative patient anatomy are insufficient to avoid complications. By combining pre-operative CT imaging with advanced computer simulations, TAVIguide technology can predict how a certain medical device will interact with a specific patient. Based on these insights, the physician gets a better view on the device-patient interaction, prior to the intervention, thus allowing optimal personalized device selection and risk management.
For more information: www.feops.com


 December 24, 2025
December 24, 2025 









