May 13, 2013 — Biosense Webster Inc. announced the first patient has been enrolled in the reMARQable clinical study. ReMARQable will assess the safety and efficacy of the use of the nMARQ Pulmonary Vein Isolation System to treat paroxysmal atrial fibrillation. Atrial fibrillation (Afib) is a heart rhythm disorder that affects approximately 20 million people worldwide.
Texas Cardiac Arrhythmia Institute (TCAI) at St. David’s Medical Center was first facility in the United States to use the nMARQ System to treat paroxysmal Afib as part of the clinical study. Andrea Natale, M.D., FHRS, FACC, FESC, cardiac electrophysiologist and executive medical director of TCAI, performed the first case in the United States, and was among the first to use this new catheter in Europe. “I am encouraged by the possibilities of this new system,” said Natale. “This technology could provide another tool to more effectively treat patients with paroxysmal Afib.”
“We are excited about the start of the pivotal U.S. clinical trial for this innovative new technology,” said Shlomi Nachman, worldwide president, Biosense Webster, Inc. “By combining the multi-ablation capability of nMARQ System with the proven irrigation technology and the unparalleled mapping accuracy of the CARTO 3 System, this new platform has been designed to reduce procedure time and complexity.”
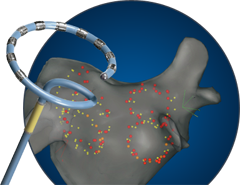
The nMARQ System consists of a multi-electrode, irrigated catheter ablation system. Each nMARQ Catheter has 10 irrigation holes per electrode completely surrounding the electrodes. The reMARQable trial will enroll approximately 400 patients at up to 50 sites in the U.S. The study is being conducted under a U.S. Food and Drug Administration (FDA)-approved Investigational Device Exemption, and the new catheter is not available in the United States outside of the reMARQable study. Further information is available at: http://clinicaltrials.gov/ct2/show/NCT01824394?term=remarqable&rank=1.
For more information: www.biosensewebster.com


 January 22, 2026
January 22, 2026 









