April 21, 2009 - The Ohio State University Medical Center in Columbus has successfully completed the first two implants of Sunshine Heart's C-Pulse Heart Assist System under a 20-patient clinical trial approved by the FDA.
Benjamin Sun, M.D., chief, division of cardiothoracic surgery and director, cardiac transplantation and mechanical support implanted the C-Pulse systems.
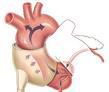
C-Pulse counterpulsation therapy involves a pliable, inflatable cuff that encircles the aorta and reportedly gently inflates and deflates in time to the patient’s native heartbeat.
"It is an honor to have completed the first U.S. implants of the C-Pulse heart assist system," said Dr. Sun. "C-Pulse is highly innovative and implanted with a simple, low-risk minimally invasive surgical procedure. The device has the potential to offer a new therapy option for the treatment of moderate heart failure."
The 20-patient FDA-approved feasibility clinical trial is being undertaken at six U.S. medical institutions: Northwestern Memorial Hospital, The Ohio State Medical Center Jewish Hospital University (University of Louisville), Hershey Medical Center of the Pennsylvania State University, University of Florida School of Medicine and University of Alabama/ Birmingham Medical Center.
For further information: www.sunshineheart.com


 June 19, 2024
June 19, 2024 









