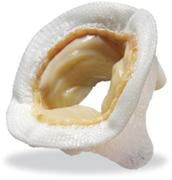
March 1, 2011 – A bioprosthetic heart valve has demonstrated positive performance at 12 years, according to a study published in The Journal of Thoracic and Cardiovascular Surgery. The Mosaic heart valve, from Medtronic, was evaluated in patients who had aortic valve replacement (AVR) and mitral valve replacement (MVR).
The study found that the device was free of structural valve deterioration in 93.3 percent of AVR patients 60 years and older and in 95.3 percent of MVR patients 70 years and older. In addition, hemodynamic performance data showed stability up to 10 years, indicating durability of the bioprosthesis over time.
The patients in this study represent a subset of patients from the original pre?market approval (PMA) trial in the United States. They were followed for 12 years at six international centers in this prospective, nonrandomized trial. The population of the present study involved 1,029 patients who received the Medtronic Mosaic porcine bioprostheses between 1994 and 2000, including 797 patients (mean age of 69 years) who had AVR and 232 patients (mean age of 67) who had MVR.
?These strong findings address and support the intermediate? to long?term durability of the Medtronic Mosaic bioprosthesis in the aortic and mitral position,? said Eric Jamieson, M.D., professor of surgery from the University of British Columbia in Vancouver, British Columbia, and a principal investigator of the study since 1994. ?Until now, there has been limited published documentation of the durability of this newer generation of bioprosthesis. I expect these results will be reviewed favorably by cardiothoracic surgeons, especially for patients 60 years of age and older requiring aortic valve replacement and patients 70 years of age and older who are candidates for mitral valve replacement.?
The bioprosthetic heart valve, approved by the U.S. Food and Drug Administration (FDA) in 2000, is an artificial heart valve used to replace a patient’s diseased or damaged natural heart valve. Usually it replaces the aortic or mitral valve, depending on a patient’s disease condition. The third?generation valve is made of porcine (pig) tissue that is attached to a cloth?covered, flexible plastic stent. It was designed for tissue preservation and calcium mitigation.
For more information: www.medtronic.com


 January 15, 2026
January 15, 2026