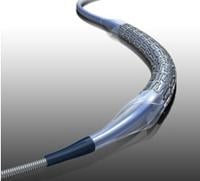
August 10, 2011 – The U.S. Food and Drug Administration (FDA) has approved Abbott’s RX Herculink Elite Renal Stent System for the treatment of renal artery stenosis (narrowing of the main arteries supplying blood to the kidneys) in patients with uncontrolled hypertension. Over time, narrowed kidney arteries can lead to kidney failure and increased risk of heart disease, stroke and peripheral artery disease. This approval is supported by the HERCULES (Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety) study, which demonstrated that RX Herculink Elite is safe and effective in patients with renal artery stenosis and uncontrolled hypertension.
"One striking result of the HERCULES study was the reduction in blood pressure we saw with RX Herculink Elite in patients with uncontrolled hypertension – those who are not adequately managed with multiple blood pressure medications," said Michael R. Jaff, D.O., medical director of the Vascular Center and VasCore, the Vascular Ultrasound Core Laboratory that participated in the HERCULES trial, both at the Massachusetts General Hospital in Boston. "This result supports renal stenting as an important treatment option, as elevations in blood pressure can increase heart disease and stroke risk in patients with renal artery stenosis."
RX Herculink Elite is the first stent using cobalt chromium technology to gain a renal indication in the United States. The cobalt chromium alloy allows for thin stent struts, providing increased flexibility while maintaining strength to support the vessel and visibility during a stent implantation procedure for accurate placement.
HERCULES is a prospective study designed to evaluate the safety and effectiveness of the RX Herculink Elite Renal Stent System in patients with atherosclerotic renal artery stenosis with uncontrolled hypertension. The study, which enrolled 202 patients at 37 study sites in the United States, met its primary endpoint, with a significantly low restenosis (vessel re-narrowing) rate of 10.5 percent at nine months post-treatment (p<0.0001). Very low safety event rates were seen, with 99 percent of patients experiencing no kidney-related safety complications within 30 days after treatment. A reduction in systolic blood pressure was seen in approximately 78 percent of patients at nine months, with the greatest reduction seen in patients who had the highest blood pressure levels at the start of the study (94 percent of patients with baseline systolic blood pressure ?180 mmHg experienced an average decrease of 48 mmHg).
"The strong results from HERCULES, particularly the notable low restenosis rate and beneficial impact on blood pressure, support renal artery stenting as an important treatment option that can have a major impact on patient care," said Charles A. Simonton, M.D., FACC, FSCAI, divisional vice president, medical affairs, and chief medical officer, Abbott Vascular. "FDA approval of RX Herculink Elite is an important milestone that reflects Abbott's commitment to developing and providing patients with the very best therapies to advance endovascular care."
In the last 12 months, Abbott has launched five new products in the United States and Europe to treat diseases of the periphery. RX Herculink Elite adds to Abbott's comprehensive portfolio of products to advance renal therapy, including balloon dilatation catheters and peripheral guide wires.
The RX Herculink Elite Renal Stent System is a cobalt chromium, rapid-exchange renal stent system. It is available in diameters ranging from 4 to 7 mm (including half sizes) and lengths of 12, 15 and 18 mm. RX Herculink Elite has been commercially available in Europe since 2007 for peripheral indications.
For more information: www.abbott.com


 January 05, 2026
January 05, 2026 









