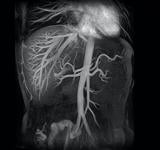
An example of a noncontrast MRA image from Toshiba.
February 26, 2010 - Gadolinium-based contrast agents, the most common contrast agents used for MRA, have been directly linked to nephrogenic systemic fibrosis or nephrogenic fibrosing dermopathy (NSF/NFD) a sometimes fatal skin disease that occurs in patients with renal insufficiency.
In response to concern over gadolinium-based contrast agents, Little Company of Mary, a nonprofit Catholic community hospital in Evergreen Park, Ill., wanted to provide the highest quality and safest MRA procedures to its patients.
To accomplish this, the facility installed a magnetic resonance imaging (MRI) system the is capable of using contrast-free MRA techniques. These contrast-free techniques allowed Little Company of Mary to now complete 98 percent of its MRA exams without contrast.
The organization acquired a Vantage Atlas MR unit by Toshiba America Medical Systems Inc. The manufacturer developed the full suite of contrast-free MRA techniques available on the entire Vantage MR product line to combat adverse medical events such as NSF/NFD. These techniques include fresh blood imaging (FBI), contrast-free tmproved angiography (CIA), time-spatial labeling inversion pulse (time-SLIP) and time and space angiography (TSA).
The system also enables the hospital to improve hospital efficiency. For example, by not using contrast the team has reduced the need for rescans that sometimes occurs when a bolus is missed, and they also do not need to run pre-exam lab tests on patients to evaluate renal function.
For more information: www.nocontrast.com


 January 21, 2026
January 21, 2026 








