September 5, 2017 — Rambam Hospital in Haifa, Israel, recently became the first to use the CORolla device from Israeli start-up company CorAssist in a 72-year-old diastolic heart failure patient.
There are several types of heart failure based on the mode of heart dysfunction. Diastolic heart failure occurs when the left ventricle fails to relax and adequately refill with blood, resulting in a high filling pressure, congestion and shortness of breath. Approximately half of heart failure patients suffer from diastolic heart failure. The incidence of diastolic heart failure increases with age and is common among women with hypertension, obesity and diabetes. There is currently no effective proven treatment for this condition.
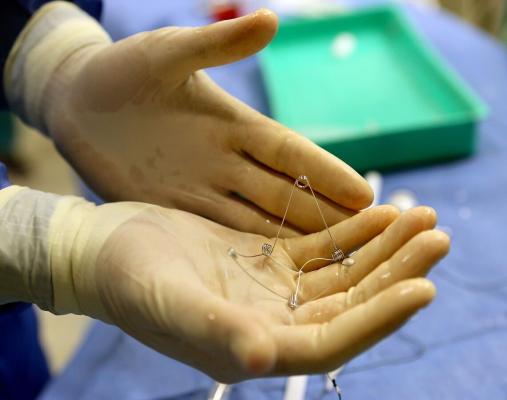
CorAssist developed the CORolla, an elastic device that is implanted inside the left ventricle of the heart by a minimally invasive procedure on a beating heart. The device can improve cardiac diastolic function by applying direct expansion force on the ventricle wall to help the heart fill with blood. The CorAssist technology was invented by Yair Feld, M.D., Ph.D., a cardiologist at Rambam Health Care Campus, together with partners Yotam Reisner, M.D., Ph.D., and Shay Dubi, M.D., Ph.D.
Prof. Gil Bolotin, director of the Department of Cardiac Surgery, and Dr. Arthur Kerner, senior physician in the Interventional Cardiology Unit, recently led a multi-disciplinary team of cardiologists, heart surgeons and other Rambam medical professionals in the first clinical implantation surgery on the 72-year old Canadian man admitted specifically for this procedure.
When asked how and why he came to Rambam for the procedure, Robert MacLachlan explained that he had run out of treatment options in Canada for his diastolic heart failure. His wife had read about the CORolla implant on the Internet and contacted Dr. Karen Bitton Worms, head of research – Department of Cardiac Surgery at Rambam. MacLachlan's cardiologist encouraged him to apply. Bolotin explained that while many potential applicants were interested in the procedure, no one wanted to be first.
The Israel Ministry of Health has authorized up to 10 clinical trials at Rambam in Israel to test the efficacy of cardiac catheterization for placement of the CORolla implant.
Watch a video animation demonstrating how the CORolla device works.
Read the related article “Device Technologies to Reduce Heart Failure Readmissions.”
Watch the VIDEO “Technologies to Reduce Heart Failure Readmissions.”
For more information: www.corassist.com


 November 14, 2025
November 14, 2025 









