June 5, 2023 — Lexicon Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has approved INPEFA (sotagliflozin), a once-daily oral tablet to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure visit in adults with:
- heart failure or
- type 2 diabetes mellitus, chronic kidney disease, and other cardiovascular risk factors.
The broad label encompasses heart failure patients across the full range of left ventricular ejection fraction (LVEF), including preserved ejection fraction and reduced ejection fraction, and for patients with or without diabetes.
“The approval of INPEFA along with the breadth of the label, is a major milestone in Lexicon’s path to fulfilling its mission of pioneering medicines that transform patients’ lives,” said Lonnel Coats, Lexicon’s chief executive officer. “We expect this important innovation to be commercially available in the U.S. market by the end of June 2023.”
The approval is based on two randomized, double-blind, placebo-controlled Phase 3 cardiovascular outcomes studies of INPEFA in patients with heart failure or at risk of heart failure. Together, SOLOIST-WHF (Worsening Heart Failure) and SCORED enrolled almost 12,000 patients. Results from SOLOIST-WHF showed that INPEFA significantly reduced risk of the composite of hospitalizations for heart failure, urgent visits for heart failure, and cardiovascular death by 33% compared to placebo in patients who had been recently hospitalized for worsening heart failure.
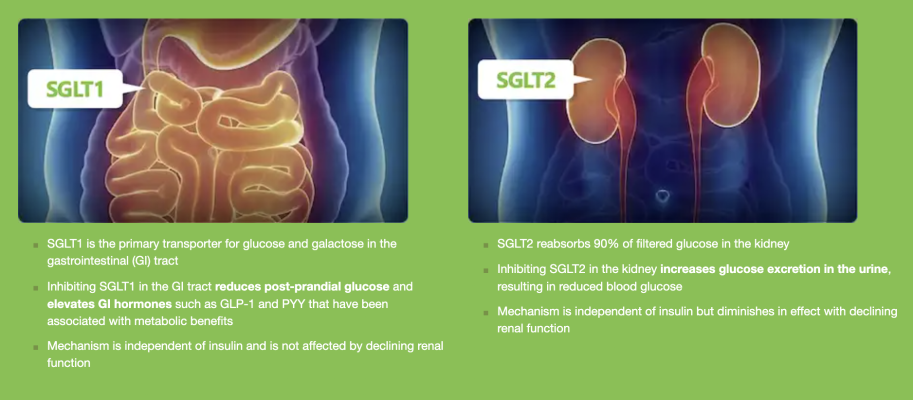
INPEFA is an inhibitor of both sodium-glucose co-transporter type 2 (SGLT2) and type 1 (SGLT1). The SGLT inhibitor class was recommended as first-line treatment for heart failure by the American Heart Association (AHA), the American College of Cardiology (ACC), and the Heart Failure Society of America (HFSA) in their joint 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. An April 2023 ACC expert consensus statement highlighted the benefit of SGLT inhibitors as part of Guideline-Directed Medical Therapy (GDMT) in patients with heart failure with preserved ejection fraction (HFpEF). According to the ACC expert consensus statement, SGLT2 inhibitors should be initiated in all individuals with HFpEF who are stable during hospitalization and have no patient population contraindications.
About 6.7 million Americans suffer from heart failure, with the prevalence expected to rise to 8.0 million by 2030. Heart failure is the leading cause of hospitalizations for individuals aged 65 and older, triggering approximately 1.3 million hospitalizations a year. Patients with heart failure are at highest risk of a heart failure event in the first 30 days post-discharge, with 7% dying and 25% being rehospitalized within one month.
“Based on outcomes observed in the SOLOIST-WHF study, initiating treatment with INPEFA prior to or upon hospital discharge has the potential to reduce the burden of readmissions on patients, caregivers, providers, and health systems,” said Craig Granowitz, M.D., Ph.D., Lexicon’s senior vice president and chief medical officer. “With today’s FDA approval, INPEFA is now a valuable option for physicians to consider when treating patients transitioning out of the hospital and working to break the cycle of repeated hospitalizations.”
Lexicon expects INPEFA to be available by the end of June 2023. The company plans to set the medicine’s wholesale acquisition cost comparable to existing branded heart failure medications.
For more information: www.lexpharma.com
Related Content:
New Analyses of Sotagliflozin Showing Time to Clinical Benefit Presented at ACC23


 July 31, 2024
July 31, 2024 









