December 9, 2011 — Maquet announced Thursday it has restructured its U.S. Sales and Services Unit to meet the broader needs of customers across all areas of clinical care. As part of this restructuring, the company now offers a comprehensive disease therapy team dedicated to servicing customers who are building a hybrid operating room (OR).
An estimated 100 hospitals in the United States currently have a hybrid OR, and the number is expected to increase by 15 percent or more over the next few years. As a top investment made by hospitals today and with approximately 60 percent of all U.S. hospitals planning for one, they represent a rapidly growing market.
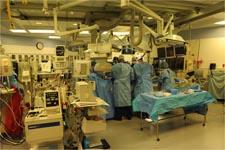
"In a hybrid OR, the cath lab doubles as an operating room so a patient undergoing a cardiac catheterization procedure can be quickly moved to surgery if required," explains Niv Ad, M.D., chief of cardiac surgery, director of cardiac surgery research, Inova Heart and Vascular Institute, Fairfax, Va. "Hybrid ORs offer the potential for improved patient outcomes, as they allow physicians to perform a full range of procedures within one setting and eliminate the need to move a critically ill patient to a different room of the hospital."
Maquet's new Hybrid Surgery Group will partner with Philips Medical and Siemens Medical to provide comprehensive therapy solutions for this growing market. These will include a surgical table that can be fully integrated with the imaging system; as well as ceiling booms; cardiac perfusion pumps; intra-aortic balloon pumps; anesthesia systems; surgical lights; and OR data integration for advanced surgical suite control.
The company has launched a new website — MAQUET-HybridOperatingRoom.com -- that provides information about planning, implementing, building and equipping a highly complex hybrid OR. The website also includes information about specific equipment and multimedia features such as a slideshow, images, videos and downloadable resources.
A hybrid OR is a state-of-the-art operating suite housing all of the equipment and monitoring devices necessary to perform open heart surgeries, such as coronary artery bypass grafting (CABG); and percutaneous coronary interventions (PCI) and procedures, including angioplasty and stenting.
Many patients suffering from coronary artery disease (CAD) can be treated less invasively in a hybrid OR, rather than going straight to a traditional operating room for CABG surgery. Containing both an OR and a cardiac catheterization lab, a hybrid OR offers an opportunity to combine minimally invasive cardiac surgery with PCI; this eliminates the need to move a critically ill patient to a different room on a different floor of the hospital.
Because hybrid ORs contain sophisticated imaging equipment, patients do not have to be sent to separate imaging suites, which is more efficient and safer for patients. They also facilitate collaboration between specialists, such as cardiologists, cardiac surgeons, interventional radiologists and vascular surgeons. The result is more efficient workflows, optimized processes and streamlined, coordinated patient care. As hybrid ORs are expected to lead to better patient outcomes and shorter lengths of stay, they could result in significant cost savings to the overall healthcare system.
For more information: www.maquet.com


 February 06, 2026
February 06, 2026 









