September 1, 2011 – Medrad Inc. has acquired Pathway Medical Technologies Inc. to strength its vascular interventional business by expanding its offers with Pathway’s mechanical atherectomy Jetstream system.
Financial terms of the agreement were not disclosed.
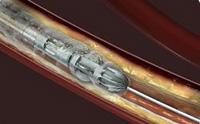
Pathway Medical Technologies's products clear blockages in leg arteries caused by peripheral artery disease (PAD). Pathway's Jetstream devices allow for a minimally invasive procedure designed to restore circulation in the peripheral arteries by reducing vascular narrowing caused by plaque. With differential cutting, Jetstream products are designed to remove plaque without harming healthy tissue.
"The combination of Medrad and Pathway Medical Technologies underscores our strategic commitment to the treatment of patients in the growing interventional field," said Jorg Reinhardt, M.D., chairman of the board of management of Bayer HealthCare, which owns Medrad. "Pathway's products complement Medrad Interventional's current and future portfolio including our injectors, thrombectomy devices and the Cotavance paclitaxel coated balloon catheter with Paccocath technology and will enable us to extend value to customers and patients through broader product options to diagnose and treat PAD."
Pathway's Jetstream alongside Bayer HealthCare's offerings in this sector create a suite of products designed to assess vascular disease, restore blood flow in diseased vessels and confirm treatment effectiveness, in support of Medrad Interventional's overall product strategy. Medrad currently offers PAD treatment with its AngioJet and Cotavance products. With the addition of Pathway's Jetstream product, Medrad offers a full range of product options for treatment of PAD.
The Cotavance catheter received CE Mark certification in Europe in 2011. MEDRAD Interventional is also moving forward with the investigational device exemption (IDE) process as one of the steps in gaining U.S. Food and Drug Administration (FDA) approval for Cotavance product in the United States.
For more information: www.pathwaymedical.com, www.medrad.com


 September 12, 2025
September 12, 2025 









