April 9, 2015 — Medtronic plc announced the first patient enrollment in the GOLD AF Registry, a prospective, observational clinical study of its Phased Radiofrequency (RF) Ablation technology for treating patients with symptomatic atrial fibrillation (AF). The multicenter registry will provide real-world insights into the procedural use and treatment outcomes of the Pulmonary Vein Ablation Catheter (PVAC) GOLD, multi-electrode ablation catheter and other catheters that comprise Medtronic's Phased RF technology.
The Medtronic Phased RF System is a generator and endocardial catheter system that delivers customized RF energy to eliminate or block abnormal electrical impulses in the left atrium that initiate or sustain AF. The system is not approved in the United States, but is under investigational device exemption (IDE) study in the VICTORY AF clinical trial.
"The GOLD AF Registry will give us the opportunity to further evaluate and uncover best practices for treating patients with Phased Radiofrequency technology," said L.V.A. Boersma, M.D., cardiologist at St. Antonius Ziekenhuis Nieuwegein, The Netherlands. "We'll be able to review data - in a very large patient cohort - on the PVAC GOLD catheter, which maps, ablates and validates pulmonary vein isolation quickly. Pulmonary vein isolation is a clinically effective therapy for treating symptomatic AF patients, and the goal of the PVAC GOLD catheter is to allow physicians to effectively isolate the veins."
The GOLD AF Registry will assess treatment by PVAC GOLD ablation of patients with paroxysmal (occasional) or persistent AF, lone AF or AF with underlying disease, and provide data on acute and mid-term success rates, as well as other procedural details and patient characteristics. It will enroll up to 1,000 patients at 50 sites in 11 countries. Patients will be observed for 12 months following a Phased RF procedure.
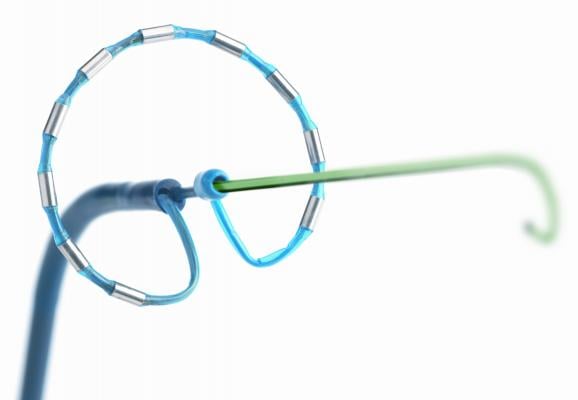
The PVAC GOLD catheter features improved thermal efficiency of gold electrodes, compared to the platinum electrodes of the first-generation system, to help physicians optimize RF ablation therapy. By delivering RF energy through nine electrodes (either all simultaneously or a subset of electrodes), the PVAC GOLD catheter helps physicians safely and efficiently create consistent lesions (intentional scar in the tissue) to effectively block the erratic AF electrical currents coming from the pulmonary veins. The gold electrodes provide four times the thermal conductivity of their platinum predecessors, offering the potential for more accurate temperature measurement and improved power delivery.
The catheter features an over-the-wire design and an anatomic shape to help physicians safely position and stabilize the catheter in different patient anatomies. A forward-tilted array helps physicians find optimal placement that is designed to improve uniformity of contact with the tissue. Following ablation, the catheter is capable of verifying PV isolation through its ability to pace and map.
For more information: www.medtronic.com


 February 06, 2026
February 06, 2026 









