August 2, 2013 — Mercator MedSystems has been issued U.S. Patent 8,465,752, which protects the company’s sole U.S. rights to deliver drugs or other nerve modifying agents to tissue surrounding arteries for renal denervation (RDN). The system also can be used for carotid body modulation (CBM) procedures.
These procedures, used to modulate or deaden hyperactive nerves, are designed to decrease uncontrolled hypertension, or high blood pressure, and are believed to be effective alternative therapies for the more than 12 million patients worldwide whose blood pressure remains uncontrolled – even with the use of three or more anti-hypertensive medications.
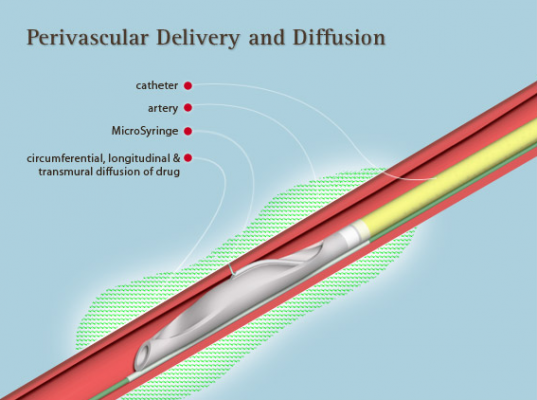
Mercator’s Micro-Infusion devices are currently used for treating peripheral artery disease (PAD), other cardiovascular diseases and pulmonary disease, and for the delivery of stem cells.
Mercator recently completed a clinical feasibility study at St. Catherine’s Hospital in Frankfurt, Germany to determine the effectiveness of lidocaine delivery, prior to energy-based renal denervation, as a means of alleviating or minimizing pain experienced with these procedures. Ablating or cauterizing the nerves lining the renal arteries with radio-frequency or ultrasound-equipped catheters often produces intense pain that is difficult to control – even with the use of systemic painkillers and morphine. Four patients were pre-treated with promising results.
After the procedures, one of St. Catherine’s treating physicians, Horst Sievert commented said, “I am impressed with the ease of delivery and the interactive visualization offered by Mercator’s Bullfrog Micro-Infusion device. While there is more work to be done, this appears to be a promising approach as a pre-treatment – and longer term, with a neuro-modulating drug, as an alternative approach to RDN.”
Mercator’s Bullfrog and Cricket Micro-Infusion devices are cleared for commercialization in Europe, the United States and Australia. The Micro-Infusion approach was created in the labs of The University of California, Berkeley in 2000.
For more information: www.mercatormed.com


 September 09, 2025
September 09, 2025 









