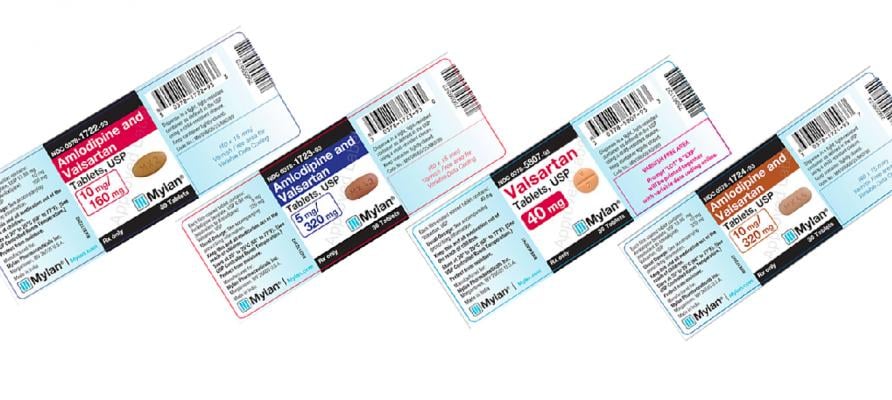
December 11, 2018 — Mylan Pharmaceuticals is expanding its consumer-level voluntary nationwide recall to include all lots of products containing Valsartan within expiry due to an impurity that has been classified as a probable human carcinogen. Valsartan is used for the treatment of high blood pressure for the treatment of heart failure, and to reduce cardiovascular mortality following myocardial infarction.
The 104 additional lots include:
-
Twenty-six (26) lots of Amlodipine and Valsartan Tablets, USP (including the 5mg/160mg, 10mg/160mg, 5mg/320mg and 10mg/320mg strengths);
-
Fifty-one (51) lots of Valsartan Tablets, USP (including 40 mg, 80 mg, 160 mg and 320 mg strengths); and
-
Twenty-seven (27) lots of Valsartan and Hydrochlorothiazide Tablets, USP (80mg/12.5mg, 160mg/12.5mg, 160mg/25mg, 320mg/12.5mg and 320mg/25mg strengths).
Valsartan in combination with amlodipine or hydrochlorothiazide is used for the treatment of high blood pressure. Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication. Patients who are on valsartan should continue taking their medication, as the risk of harm to the patient’s health may be higher if the treatment is stopped immediately without any alternative treatment.
These products are being recalled due to detected trace amounts of an impurity, N-nitrosodiethylamine (NDEA) contained in the API Valsartan, USP, manufactured by Mylan Laboratories Ltd. NDEA is a substance that occurs naturally in certain foods, drinking water, air pollution and industrial processes, and has been classified as a probable human carcinogen according to the International Agency for Research on Cancer (IARC).
The finished products are manufactured by Mylan Pharmaceuticals Inc. and Mylan Laboratories Ltd. These batches were distributed in the U.S. between March 2017 and November 2018.
Mylan is notifying its distributors and customers by letter and is arranging for return of all recalled products. Wholesalers, retailers and consumers that are in possession of recalled product should contact Stericycle at 1-888-406-9305 for the return of the recalled product. Normal business hours are Monday through Friday 8 a.m. to 5 p.m. EST.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
-
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax. Complete and submit the report Online: www.fda.gov/medwatch/report.htm
-
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
The full expanded list of recalled batches can be seen here.
For more information: www.fda.gov


 September 09, 2025
September 09, 2025 









