December 29, 2016 — The American College of Cardiology (ACC), along with several partnering organizations, recently released updated appropriate use criteria for performing coronary revascularization in patients with acute coronary syndromes.
Patients with acute coronary syndromes suffer from sudden, reduced blood flow to the heart, requiring quick diagnosis and care. Coronary revascularization is the restoration of this blood flow to the heart commonly by coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI).
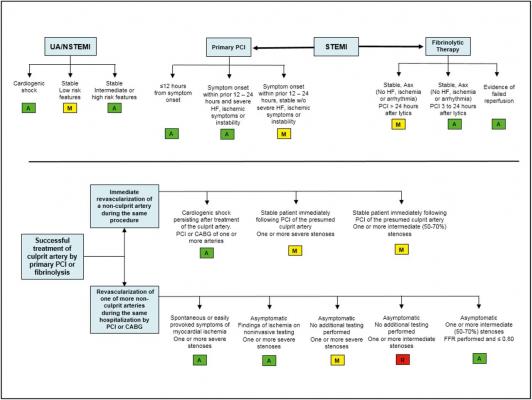
The appropriate use criteria document includes clinical scenarios developed to mimic patient presentations that may be encountered in everyday practice and information on symptom status, presence of clinical instability or ongoing ischemic symptoms, prior reperfusion therapy, risk level as assessed by noninvasive testing, fractional flow reserve testing, and coronary anatomy.
“This update provides a reassessment of clinical scenarios that the writing group felt to be affected by significant changes in the medical literature or gaps from prior criteria,” said Manesh R. Patel, M.D., FACC, FAHA, FSCAI, chief of the division of cardiology and co-director of the Duke Heart Center at Duke University and chair of the writing committee for the document. “The primary objective of the appropriate use criteria is to provide a framework for the assessment of practice patterns that will hopefully improve physician decision making and ultimately lead to better patient outcomes.”
The clinical scenarios are scored to indicate whether revascularization is appropriate, may be appropriate or is rarely appropriate for the clinical scenario presented.
The document can be used as a clinical tool to assist clinicians in evaluating therapies and can help to better inform patients about their treatment options. It is important for patients to discuss revascularization and engage in shared decision making with their provider to come to a decision on the best treatment plan.
The writing committee stresses that the criteria should be used as an overall guide, and physicians should evaluate each case on an individual basis.
These appropriate use criteria are a product of a partnership between the ACC, the American Association for Thoracic Surgery, the American Heart Association, American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society for Cardiovascular Angiography and Interventions, the Society of Cardiovascular Computed Tomography and the Society of Thoracic Surgeons. The document is part one of a two-part revision for coronary revascularization. The updated appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease are forthcoming.
The "ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2016 Appropriate Use Criteria for Coronary Revascularization in Patients With Acute Coronary Syndromes" was published online Dec. 21 in the Journal of the American College of Cardiology.
For more information: acc.org, ASEcho.org, www.asnc.org, www.SCAI.org, www.scct.org


 July 31, 2024
July 31, 2024 









